A Flow Cytometric Method for Isolating Cystic Fibrosis Airway Macrophages from Expectorated Sputum
- PMID: 30742539
- PMCID: PMC6604218
- DOI: 10.1165/rcmb.2018-0236MA
A Flow Cytometric Method for Isolating Cystic Fibrosis Airway Macrophages from Expectorated Sputum
Abstract
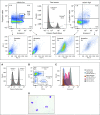
Research to understand the contribution of macrophages to nonresolving airway inflammation in cystic fibrosis (CF) and other chronic suppurative airways diseases has been hindered by a lack of methods for isolating and studying these cells. With the development of technologies that can characterize small numbers of cells or individual cells, there is an even greater need for methodologies to isolate rare cells in heterogeneous specimens. Here, we describe a method that overcomes the technical obstacles imposed by sputum debris and apoptotic cells, and allows isolation of pure populations of macrophages from CF sputum. In addition to enhancing our ability to study human CF airway macrophages, this protocol can be adapted to study cells in sputum from other chronic suppurative lung diseases (e.g., chronic obstructive pulmonary disease) and used for isolation of individual cells for single cell analyses.
Keywords: cystic fibrosis; macrophages; single-cell analyses; sputum.
Figures


References
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med. 2003;168:918–951. - PubMed
-
- Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

