The master quorum-sensing regulators LuxR/HapR directly interact with the alpha subunit of RNA polymerase to drive transcription activation in Vibrio harveyi and Vibrio cholerae
- PMID: 30742725
- PMCID: PMC6488372
- DOI: 10.1111/mmi.14223
The master quorum-sensing regulators LuxR/HapR directly interact with the alpha subunit of RNA polymerase to drive transcription activation in Vibrio harveyi and Vibrio cholerae
Abstract
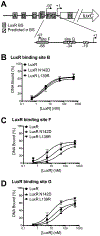
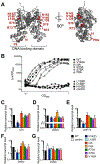
In Vibrio species, quorum sensing controls gene expression for numerous group behaviors, including bioluminescence production, biofilm formation, virulence factor secretion systems, and competence. The LuxR/HapR master quorum-sensing regulators activate expression of hundreds of genes in response to changes in population densities. The mechanism of transcription activation by these TetR-type transcription factors is unknown, though LuxR DNA binding sites that lie in close proximity to the -35 region of the promoter are required for activation at some promoters. Here, we show that Vibrio harveyi LuxR directly interacts with RNA polymerase to activate transcription of the luxCDABE bioluminescence genes. LuxR interacts with RNA polymerase in vitro and in vivo and specifically interacts with both the N- and C-terminal domains of the RNA polymerase α-subunit. Amino acid substitutions in the RNAP interaction domain on LuxR decrease interactions between LuxR and the α-subunit and result in defects in transcription activation of quorum-sensing genes in vivo. The RNAP-LuxR interaction domain is conserved in Vibrio cholerae HapR and is required for activation of the HapR-regulated gene hapA. Our findings support a model in which LuxR/HapR bind proximally to RNA polymerase to drive transcription initiation at a subset of quorum-sensing genes in Vibrio species.
© 2019 John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare that that they have no conflicts of interest to declare.
Figures

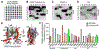

Similar articles
-
Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios.J Bacteriol. 2017 Sep 5;199(19):e00105-17. doi: 10.1128/JB.00105-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28484045 Free PMC article. Review.
-
Hierarchical Transcriptional Control of the LuxR Quorum-Sensing Regulon of Vibrio harveyi.J Bacteriol. 2020 Jun 25;202(14):e00047-20. doi: 10.1128/JB.00047-20. Print 2020 Jun 25. J Bacteriol. 2020. PMID: 32366592 Free PMC article.
-
Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi.Mol Microbiol. 2016 Sep;101(5):823-40. doi: 10.1111/mmi.13425. Epub 2016 Jun 16. Mol Microbiol. 2016. PMID: 27191515
-
AphA and LuxR/HapR reciprocally control quorum sensing in vibrios.Genes Dev. 2011 Feb 15;25(4):397-408. doi: 10.1101/gad.2015011. Genes Dev. 2011. PMID: 21325136 Free PMC article.
-
Role and regulation of bacterial LuxR-like regulators.J Cell Biochem. 2011 Oct;112(10):2694-702. doi: 10.1002/jcb.23219. J Cell Biochem. 2011. PMID: 21678467 Review.
Cited by
-
HilD induces expression of a novel Salmonella Typhimurium invasion factor, YobH, through a regulatory cascade involving SprB.Sci Rep. 2019 Sep 4;9(1):12725. doi: 10.1038/s41598-019-49192-z. Sci Rep. 2019. PMID: 31484980 Free PMC article.
-
Quorum-Sensing Regulator OpaR Directly Represses Seven Protease Genes in Vibrio parahaemolyticus.Front Microbiol. 2020 Oct 29;11:534692. doi: 10.3389/fmicb.2020.534692. eCollection 2020. Front Microbiol. 2020. PMID: 33193123 Free PMC article.
-
GplR1, an unusual TetR-like transcription factor in Mycobacterium abscessus, controls the production of cell wall glycopeptidolipids, colony morphology, and virulence.bioRxiv [Preprint]. 2025 May 8:2025.05.07.652718. doi: 10.1101/2025.05.07.652718. bioRxiv. 2025. PMID: 40654710 Free PMC article. Preprint.
-
Overcoming stochastic variations in culture variables to quantify and compare growth curve data.Bioessays. 2021 Aug;43(8):e2100108. doi: 10.1002/bies.202100108. Epub 2021 Jun 14. Bioessays. 2021. PMID: 34128245 Free PMC article.
-
Purification of the Vibrio Quorum-Sensing Transcription Factors LuxR, HapR, and SmcR.Methods Mol Biol. 2021;2346:173-182. doi: 10.1007/7651_2020_306. Methods Mol Biol. 2021. PMID: 32705543
References
-
- Bokal A.J.t., Ross W & Gourse RL, (1995) The transcriptional activator protein FIS: DNA interactions and cooperative interactions with RNA polymerase at the Escherichia coli rrnB P1 promoter. J Mol Biol 245: 197–207. - PubMed
-
- Chaparian RR, Olney SG, Hustmyer CM, Rowe-Magnus DA & van Kessel JC, (2016) Integration host factor and LuxR synergistically bind DNA to coactivate quorum-sensing genes in Vibrio harveyi. Mol Microbiol 101: 823–840. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

