Cross-Isotopic Bioorthogonal Tools as Molecular Twins for Radiotheranostic Applications
- PMID: 30742739
- PMCID: PMC6617999
- DOI: 10.1002/cbic.201900042
Cross-Isotopic Bioorthogonal Tools as Molecular Twins for Radiotheranostic Applications
Abstract
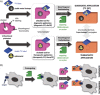
Radiotheranostics are designed by labeling targeting (bio)molecules with radionuclides for diagnostic or therapeutic application. Because the pharmacokinetics of therapeutic compounds play a pivotal role, chemically closely related imaging agents are used to evaluate the overall feasibility of the therapeutic approach. "Theranostic relatives" that utilize different elements are frequently used in clinical practice. However, variations in pharmacokinetics, biodistribution, and target affinity due to different chemical properties of the radioisotopes remain as hurdles to the design of optimized clinical tools. Herein, the design and synthesis of structurally identical compounds, either for diagnostic (18 F and a stable metal isotope) or therapeutic application (radiometal and stable 19 F), are reported. Such "molecular twins" have been prepared by applying a modular strategy based on click chemistry that enables efficient radiolabeling of compounds containing a metal complex and a tetrazine moiety. This additional bioorthogonal functionality can be used for subsequent radiolabeling of (bio)molecules or pretargeting approaches, which is demonstrated in vitro.
Keywords: bioorthogonal chemistry; click chemistry; isotopes; radiochemistry; radiopharmaceuticals.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
Figures





References
-
- Curie P., Skłodowska Curie M., Bémont G., C. R. Acad. Sci. 1898, 127, 1215–1215.
-
- Colombo I., Overchuk M., Chen J., Reilly R. M., Zheng G., Lheureux S., Methods 2017, 130, 23–35. - PubMed
-
- van Rij C. M., Sharkey R. M., Goldenberg D. M., Frielink C., Molkenboer J. D. M., Franssen G. M., van Weerden W. M., Oyen W. J. G., Boerman O. C., J. Nucl. Med. 2011, 52, 1601–1607. - PubMed
-
- Fani M., Maecke H. R., Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 11–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

