Optimization of qRT-PCR assay for zika virus detection in human serum and urine
- PMID: 30742853
- PMCID: PMC6561482
- DOI: 10.1016/j.virusres.2019.01.013
Optimization of qRT-PCR assay for zika virus detection in human serum and urine
Abstract
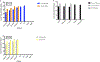
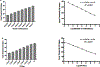
Zika Virus (ZIKV) is a mosquito-borne flavivirus that the World Health Organization (WHO) declared a global concern due to the severity of infection. This study focuses on determining the level of detection of ZIKV RNA in human serum and urine. Known amounts of Zika virus were added to uninfected human serum and urine samples. Different reverse transcriptases were compared to select the optimal enzyme for this application. Zika RNA in these samples was then quantified with qRT-PCR to determine the lower limit of detection in these fluids and to construct a standard curve. Student's t-test of paired samples was used in order to identify statistical differences. The SuperScript III enzyme was able to produce more ZIKV cDNA when compared to PrimeScript. Zika virus RNA was found to be detectable at lower levels (2.5 PFU/mL) in urine than in serum (250 PFU/mL) when using SuperScript III. This study demonstrates how the selection of both the human clinical specimen, and the reverse transcriptase enzyme involved in the molecular detection of ZIKV by quantitative real-time polymerase chain reaction (qRT-PCR), play an important role in enabling improved detection of the virus.
Keywords: Clinical samples; Limit of detection (LOD); Reverse transcriptase; Zika Virus (ZIKV); qRT-PCR.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflict of interest.
Figures

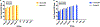
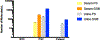
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

