The molecular chaperone sigma 1 receptor mediates rescue of retinal cone photoreceptor cells via modulation of NRF2
- PMID: 30743048
- PMCID: PMC6619428
- DOI: 10.1016/j.freeradbiomed.2019.02.001
The molecular chaperone sigma 1 receptor mediates rescue of retinal cone photoreceptor cells via modulation of NRF2
Abstract
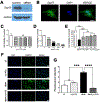
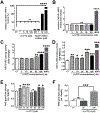
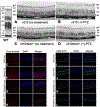
Sigma 1 receptor (Sig1R), a putative molecular chaperone, has emerged as a novel therapeutic target for retinal degenerative disease. Earlier studies showed that activation of Sig1R via the high-affinity ligand (+)-pentazocine ((+)-PTZ) induced profound rescue of cone photoreceptor cells in the rd10 mouse model of retinitis pigmentosa; however the mechanism of rescue is unknown. Improved cone function in (+)-PTZ-treated mice was accompanied by reduced oxidative stress and normalization of levels of NRF2, a transcription factor that activates antioxidant response elements (AREs) of hundreds of cytoprotective genes. Here, we tested the hypothesis that modulation of NRF2 is central to Sig1R-mediated cone rescue. Activation of Sig1R in 661W cone cells using (+)-PTZ induced dose-dependent increases in NRF2-ARE binding activity and NRF2 gene/protein expression, whereas silencing Sig1R significantly decreased NRF2 protein levels and increased oxidative stress, although (+)-PTZ did not disrupt NRF2-KEAP1 binding. In vivo studies were conducted to investigate whether, in the absence of NRF2, activation of Sig1R rescues cones. (+)-PTZ was administered systemically for several weeks to rd10/nrf2+/+ and rd10/nrf2-/- mice. Through post-natal day 42, cone function was significant in rd10/nrf2+/+, but minimal in rd10/nrf2-/- mice as indicated by electroretinographic recordings using natural noise stimuli, optical coherence tomography and retinal histological analyses. Immunodetection of cones was limited in (+)-PTZ-treated rd10/nrf2-/-, though considerable in (+)-PTZ-treated rd10/nrf2+/+mice. The data suggest that Sig1R-mediated cone rescue requires NRF2 and provide evidence for a previously-unrecognized relationship between these proteins.
Keywords: NRF2-KEAP1; NRF2-Neh luciferase assay; Oxidative stress; Retina; Retinal neuroprotection; Retinitis pigmentosa; rd10 mouse.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Comparison of Neuroprotective Effects of Monomethylfumarate to the Sigma 1 Receptor Ligand (+)-Pentazocine in a Murine Model of Retinitis Pigmentosa.Invest Ophthalmol Vis Sci. 2020 Mar 9;61(3):5. doi: 10.1167/iovs.61.3.5. Invest Ophthalmol Vis Sci. 2020. PMID: 32150247 Free PMC article.
-
Activation of Sigma 1 Receptor Extends Survival of Cones and Improves Visual Acuity in a Murine Model of Retinitis Pigmentosa.Invest Ophthalmol Vis Sci. 2019 Oct 1;60(13):4397-4407. doi: 10.1167/iovs.19-27709. Invest Ophthalmol Vis Sci. 2019. PMID: 31639826 Free PMC article.
-
A Novel Mechanism of Sigma 1 Receptor Neuroprotection: Modulation of miR-214-3p.Adv Exp Med Biol. 2019;1185:463-467. doi: 10.1007/978-3-030-27378-1_76. Adv Exp Med Biol. 2019. PMID: 31884655
-
Sigma 1 receptor: A novel therapeutic target in retinal disease.Prog Retin Eye Res. 2018 Nov;67:130-149. doi: 10.1016/j.preteyeres.2018.07.003. Epub 2018 Aug 1. Prog Retin Eye Res. 2018. PMID: 30075336 Free PMC article. Review.
-
Nrf2 protects against airway disorders.Toxicol Appl Pharmacol. 2010 Apr 1;244(1):43-56. doi: 10.1016/j.taap.2009.07.024. Epub 2009 Jul 29. Toxicol Appl Pharmacol. 2010. PMID: 19646463 Review.
Cited by
-
Cytotoxicity Profiles and Neuroprotective Properties of the Novel Ifenprodil Analogues as Sigma Ligands.Molecules. 2023 Apr 13;28(8):3431. doi: 10.3390/molecules28083431. Molecules. 2023. PMID: 37110664 Free PMC article.
-
Chronic Sigma 1 receptor activation alleviates right ventricular dysfunction secondary to pulmonary arterial hypertension.Bioengineered. 2022 Apr;13(4):10843-10856. doi: 10.1080/21655979.2022.2065953. Bioengineered. 2022. PMID: 35473584 Free PMC article.
-
Evaluation of the role of Sigma 1 receptor and Cullin3 in retinal photoreceptor cells.Free Radic Biol Med. 2023 Aug 20;205:214-223. doi: 10.1016/j.freeradbiomed.2023.06.010. Epub 2023 Jun 14. Free Radic Biol Med. 2023. PMID: 37328017 Free PMC article.
-
Comparison of Sigma 1 Receptor Ligands SA4503 and PRE084 to (+)-Pentazocine in the rd10 Mouse Model of RP.Invest Ophthalmol Vis Sci. 2020 Nov 2;61(13):3. doi: 10.1167/iovs.61.13.3. Invest Ophthalmol Vis Sci. 2020. PMID: 33137196 Free PMC article.
-
Targeting the sigma-1 receptor with pridopidine induces functional neurorestoration in spinal cord ischemia-reperfusion injury.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):9307-9321. doi: 10.1007/s00210-025-03851-3. Epub 2025 Feb 12. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39937253 Free PMC article.
References
-
- Tadić V, Malci A, Goldhammer N, Stubendorff B, Sengupta S, Prell T, Keiner S, Liu J, Guenther M, Frahm C, Witte OW, Grosskreutz J. Sigma 1 receptor activation modifies intracellular calcium exchange in the G93A(hSOD1) ALS model. Neuroscience. 359:105–118;2017. - PubMed
-
- Mavlyutov TA, Baker EM, Losenegger TM, Kim JR, Torres B, Epstein ML, Ruoho AE. The Sigma-1 Receptor-A Therapeutic Target for the Treatment of ALS? Adv Exp Med Biol. 964:255–265;2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

