Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease
- PMID: 30743314
- PMCID: PMC6533444
- DOI: 10.1111/bcp.13896
Pharmacokinetics and pharmacodynamics of voxelotor (GBT440) in healthy adults and patients with sickle cell disease
Abstract
Aims: Voxelotor (previously GBT440) is a haemoglobin (Hb) modulator that increases Hb-oxygen affinity, thereby reducing Hb polymerization and sickling of red blood cells (RBCs), being developed as a once-daily oral drug to treat sickle cell disease (SCD). This first-in-human study evaluated the safety, tolerability, pharmacokinetics and pharmacodynamics of voxelotor in healthy volunteers and SCD patients.
Methods: A total of 40 healthy volunteers (100, 400, 1000, 2000 or 2800 mg) and 8 SCD patients (1000 mg) were randomly assigned to a single dose of voxelotor once daily (n = 6 per group) or placebo (n = 2 per group). Twenty-four healthy volunteers received multiple doses of voxelotor once daily for 15 days (300, 600 or 900 mg, n = 6 per group) or placebo (n = 2 per group).
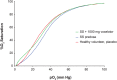
Results: Voxelotor was well tolerated and exhibited a linear pharmacokinetic profile and a half-life ranging from 61 ± 7 h to 85 ± 7 h. High partitioning into the RBC compartment provides evidence of highly specific binding to Hb. Voxelotor exhibited a concentration-dependent left-shift of oxygen equilibrium curves. Percent Hb modification following 900 mg voxelotor for 15 days was 38 ± 9%. Terminal half-life of voxelotor in SCD patients (50 ± 3 h) was shorter than in healthy volunteers. Evaluation of erythropoietin, exercise testing, and haematologic parameters were consistent with normal oxygen delivery during both rest and exercise.
Conclusion: This first-in-human study demonstrates voxelotor was well tolerated in SCD patients and healthy volunteers and established proof of mechanism on increasing Hb-oxygen affinity.
Keywords: clinical trial; pharmacodynamics; pharmacokinetics; phase I; sickle cell disease; voxelotor.
© 2019 Global Blood Therapeutics. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
A.H., M.P., C.W., V.S., D.O. and J.L.‐G. are employees of and have equity ownership in Global Blood Therapeutics. T.M. and E.A. are employees of IQVIA. T.M. owns shares of IQVIA. D.D.G. is an independent consultant.
Figures






Similar articles
-
A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease.Blood. 2019 Apr 25;133(17):1865-1875. doi: 10.1182/blood-2018-08-868893. Epub 2019 Jan 17. Blood. 2019. PMID: 30655275 Free PMC article. Clinical Trial.
-
A Phase 3 Randomized Trial of Voxelotor in Sickle Cell Disease.N Engl J Med. 2019 Aug 8;381(6):509-519. doi: 10.1056/NEJMoa1903212. Epub 2019 Jun 14. N Engl J Med. 2019. PMID: 31199090 Clinical Trial.
-
Systematic Review of Voxelotor: A First-in-Class Sickle Hemoglobin Polymerization Inhibitor for Management of Sickle Cell Disease.Pharmacotherapy. 2020 Jun;40(6):525-534. doi: 10.1002/phar.2405. Epub 2020 May 19. Pharmacotherapy. 2020. PMID: 32343424
-
Voxelotor: A Novel Treatment for Sickle Cell Disease.Ann Pharmacother. 2021 Feb;55(2):240-245. doi: 10.1177/1060028020943059. Epub 2020 Jul 16. Ann Pharmacother. 2021. PMID: 32674605 Review.
-
Voxelotor in adolescents and adults with sickle cell disease (HOPE): long-term follow-up results of an international, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Haematol. 2021 May;8(5):e323-e333. doi: 10.1016/S2352-3026(21)00059-4. Epub 2021 Apr 7. Lancet Haematol. 2021. PMID: 33838113 Clinical Trial.
Cited by
-
Pyrazole-containing pharmaceuticals: target, pharmacological activity, and their SAR studies.RSC Med Chem. 2022 Aug 26;13(11):1300-1321. doi: 10.1039/d2md00206j. eCollection 2022 Nov 16. RSC Med Chem. 2022. PMID: 36439976 Free PMC article. Review.
-
Gene-environmental influence of space and microgravity on red blood cells with sickle cell disease.NPJ Genom Med. 2024 Sep 30;9(1):44. doi: 10.1038/s41525-024-00427-7. NPJ Genom Med. 2024. PMID: 39349487 Free PMC article. Review.
-
Biophysical chemistry behind sickle cell anemia and the mechanism of voxelotor action.Sci Rep. 2024 Jan 22;14(1):1861. doi: 10.1038/s41598-024-52476-8. Sci Rep. 2024. PMID: 38253605 Free PMC article.
-
Sickle Cell Disease: Current Drug Treatments and Functional Foods with Therapeutic Potential.Curr Issues Mol Biol. 2024 Jun 12;46(6):5845-5865. doi: 10.3390/cimb46060349. Curr Issues Mol Biol. 2024. PMID: 38921020 Free PMC article. Review.
-
Hypoxia as a medicine.Sci Transl Med. 2025 Jan 22;17(782):eadr4049. doi: 10.1126/scitranslmed.adr4049. Epub 2025 Jan 22. Sci Transl Med. 2025. PMID: 39841808 Free PMC article. Review.
References
-
- Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561‐1573. - PubMed
-
- Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762‐769. - PubMed
-
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: A 4‐decade observational study of 1056 patients. Medicine (Baltimore). 2005;84:36‐76. - PubMed
-
- Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38(4):S512‐S521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

