The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method
- PMID: 30743992
- PMCID: PMC6386965
- DOI: 10.3390/ijms20030721
The Composition of Hyperacute Serum and Platelet-Rich Plasma Is Markedly Different despite the Similar Production Method
Abstract
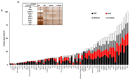
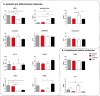
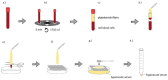
Autologous blood derived products, such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) are widely applied in regenerative therapies, in contrast to the drawbacks in their application, mainly deriving from the preparation methods used. Eliminating the disadvantages of both PRP and PRF, hyperacute serum (HAS) opens a new path in autologous serum therapy showing similar or even improved regenerative potential at the same time. Despite the frequent experimental and clinical use of PRP and HAS, their protein composition has not been examined thoroughly yet. Thus, we investigated and compared the composition of HAS, serum, PRP and plasma products using citrate and EDTA by simple laboratory tests, and we compared the composition of HAS, serum, EDTA PRP and plasma by Proteome Profiler and ELISA assays. According to our results the natural ionic balance was upset in both EDTA and citrate PRP as well as in plasma. EDTA PRP contained significantly higher level of growth factors and cytokines, especially platelet derived angiogenic and inflammatory proteins, that can be explained by the significantly higher number of platelets in EDTA PRP. The composition analysis of blood derivatives revealed that although the preparation method of PRP and HAS were similar, the ionic and protein composition of HAS could be advantageous for cell function.
Keywords: blood derived products; composition; hyperacute serum; platelet-rich plasma.
Conflict of interest statement
Z.L. owns stock in OrthoSera GmbH, a startup company developing the hyperacute serum technology towards clinical application.
Figures





References
-
- Amable P.R., Carias R.B.V., Teixeira M.V.T., da Cruz Pacheco I., Corrêa do Amaral R.J.F., Granjeiro J.M., Borojevic R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013;4:67. doi: 10.1186/scrt218. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

