Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution
- PMID: 30744025
- PMCID: PMC6410061
- DOI: 10.3390/nano9020230
Development of Solid Lipid Nanoparticles by Cold Dilution of Microemulsions: Curcumin Loading, Preliminary In Vitro Studies, and Biodistribution
Abstract
Background: Solid lipid nanoparticles (SLNs) are attractive drug delivery systems for lipophilic molecules like curcumin (CURC) with low chemical stability. Methods: A simple, innovative, and cold-operating method, named "cold dilution of microemulsion" is developed by the authors to produce SLNs. An oil-in-water microemulsion (µE), whose disperse phase consisted of a solution of trilaurin in a partially water-miscible solvent, was prepared after mutually saturating solvent and water. Trilaurin SLNs precipitated following solvent removal upon water dilution of the µE. After SLN characterization (mean size, Zeta potential, CURC entrapment efficiency, and over time stability), they were tested for in vitro cytotoxicity studies on pancreatic adenocarcinoma cell lines and for in vivo preliminary biodistribution studies in Wistar healthy rats. Results: CURC loaded SLNs (SLN-CURC) had mean diameters around 200 nm, were negatively charged, stable over time, and able to entrap CURC up to almost 90%, consequently improving its stability. SLN-CURC inhibited in vitro pancreatic carcinoma cell growth in concentration-dependent manner. Their in vivo intravenous administration suggested a possible long circulation. Conclusions: These results, according to a concomitant study on chitosan-coated SLNs, confirm the possibility to apply the developed SLN-based delivery systems as a means to entrap CURC, to improve both its water dispersibility and chemical stability, facilitating its application in therapy.
Keywords: cancer chemoprevention; cell line; drug delivery system; microemulsions; nanoparticles; poorly water-soluble drug.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
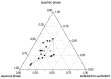
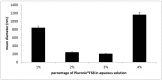
 500 nm).
500 nm).


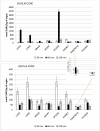
Similar articles
-
Stearoyl-Chitosan Coated Nanoparticles Obtained by Microemulsion Cold Dilution Technique.Int J Mol Sci. 2018 Nov 30;19(12):3833. doi: 10.3390/ijms19123833. Int J Mol Sci. 2018. PMID: 30513699 Free PMC article.
-
Physicochemical Characterization, Stability, and In Vitro Evaluation of Curcumin-Loaded Solid Lipid Nanoparticles Prepared Using Biocompatible Synthetic Lipids.ACS Appl Bio Mater. 2023 Jul 17;6(7):2785-2794. doi: 10.1021/acsabm.3c00252. Epub 2023 Jul 5. ACS Appl Bio Mater. 2023. PMID: 37403739
-
Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability.Colloids Surf B Biointerfaces. 2013 Nov 1;111:367-75. doi: 10.1016/j.colsurfb.2013.06.032. Epub 2013 Jun 25. Colloids Surf B Biointerfaces. 2013. PMID: 23856543
-
Improved Method for Solid Lipid Nanoparticle Preparation Based on Hot Microemulsions: Preparation, Characterization, Cytotoxicity, and Hemocompatibility Evaluation.AAPS PharmSciTech. 2017 May;18(4):1355-1365. doi: 10.1208/s12249-016-0606-z. Epub 2016 Aug 8. AAPS PharmSciTech. 2017. PMID: 27502405
-
Physicochemical characterization techniques for solid lipid nanoparticles: principles and limitations.Drug Dev Ind Pharm. 2014 Dec;40(12):1565-75. doi: 10.3109/03639045.2014.909840. Epub 2014 Apr 25. Drug Dev Ind Pharm. 2014. PMID: 24766553 Review.
Cited by
-
A New Bevacizumab Carrier for Intravitreal Administration: Focus on Stability.Pharmaceutics. 2021 Apr 15;13(4):560. doi: 10.3390/pharmaceutics13040560. Pharmaceutics. 2021. PMID: 33921167 Free PMC article.
-
Naked and Decorated Nanoparticles Containing H2S-Releasing Doxorubicin: Preparation, Characterization and Assessment of Their Antitumoral Efficiency on Various Resistant Tumor Cells.Int J Mol Sci. 2022 Sep 30;23(19):11555. doi: 10.3390/ijms231911555. Int J Mol Sci. 2022. PMID: 36232858 Free PMC article.
-
Overview of Solid Lipid Nanoparticles in Breast Cancer Therapy.Pharmaceutics. 2023 Jul 31;15(8):2065. doi: 10.3390/pharmaceutics15082065. Pharmaceutics. 2023. PMID: 37631279 Free PMC article. Review.
-
Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects.Front Mol Biosci. 2020 Oct 30;7:587997. doi: 10.3389/fmolb.2020.587997. eCollection 2020. Front Mol Biosci. 2020. PMID: 33195435 Free PMC article. Review.
-
Solid Lipid Nanoparticles: Multitasking Nano-Carriers for Cancer Treatment.Pharmaceutics. 2023 Mar 3;15(3):831. doi: 10.3390/pharmaceutics15030831. Pharmaceutics. 2023. PMID: 36986692 Free PMC article. Review.

