Stereoselectivity evaluation of chiral chitosan microspheres delivery system containing rac-KET in vitro and in vivo
- PMID: 30744429
- PMCID: PMC6374939
- DOI: 10.1080/10717544.2018.1556360
Stereoselectivity evaluation of chiral chitosan microspheres delivery system containing rac-KET in vitro and in vivo
Abstract
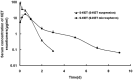
The influence of chiral excipient D-chitosan (CS) on the stereoselective release of racemic ketoprofen (rac-KET) microspheres has been investigated in comparison to those microspheres containing individual enantiomers in vitro and in vivo. Stereoselectivity was observed in vitro release test, with R-KET release slightly higher than that of S-KET, especially in 3% rac-KET loading microspheres. Stereoselectivity is dependent on the content of chiral excipient and pH of release medium. A molecular docking study between CS and KET enantiomers further revealed that S-KET has a stronger interaction with CS compared to R-KET. Moreover, the plasma concentration of KET enantiomers in rats shows substantial differences, as the plasma levels of S-KET were higher than those of R-KET. Plasma levels of enantiomers from the R-KET microspheres had similar stereoselectivity as rac-KET microspheres. The S/R ratio of rac-KET microspheres was significantly lower than that of rac-KET suspension (regular-release formulation) (p<.05), and the differences is 3-5 fold. Besides, rates of R-KET converted to S-KET exhibited differences between rac-KET microspheres and suspension. Similar results were also found between R-KET microspheres and suspension. All investigations suggest that the chitosan interacting preferentially with S-KET to R-KET significantly affect the stereoselective pharmacokinetics of rac-KET from chitosan microspheres in rats.
Keywords: Stereoselective release; chiral excipient; chitosan; ketoprofen; molecular docking study; stereoselective pharmacokinetics.
Figures


References
-
- Álvarez C, Torrado JJ, Cadórniga R (1999). Stereoselective drug release from ketoprofen and ricobendazole matrix tablets. Chirality 11:611–5. - PubMed
-
- Asghar W, Pittman E, Jamali F (2015). Most chiral drugs have been used as racemates while the beneficial effects are often attributed mainly to one of the enantiomers. DARU J Pharm Sci 23:50–7.
-
- Barbanoj MJ, Antonijoan RM, Gich I (2001). Clinical pharmacokinetics of dexketoprofen. Clin Pharmacokinet 40:245–62. - PubMed
-
- Bressolle F, Rochette A, Khier S, et al. (2009). Population pharmacokinetics of the two enantiomers of tramadol and O-demethyl tramadol after surgery in children. Brit J Anaesth 102:390–9. - PubMed
-
- Brocks DR. (2000). Stereoselective pharmacokinetics of desbutylhalofantrine, a metabolite of halofantrine, in the rat after administration of the racemic metabolite or parent drug. Biopharm Drug Dispos 21:365–71. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
