Anti-CD117 antibody depletes normal and myelodysplastic syndrome human hematopoietic stem cells in xenografted mice
- PMID: 30745302
- PMCID: PMC6509544
- DOI: 10.1182/blood-2018-06-858159
Anti-CD117 antibody depletes normal and myelodysplastic syndrome human hematopoietic stem cells in xenografted mice
Abstract
The myelodysplastic syndromes (MDS) represent a group of clonal disorders that result in ineffective hematopoiesis and are associated with an increased risk of transformation into acute leukemia. MDS arises from hematopoietic stem cells (HSCs); therefore, successful elimination of MDS HSCs is an important part of any curative therapy. However, current treatment options, including allogeneic hematopoietic cell transplantation (HCT), often fail to ablate disease-initiating MDS HSCs, and thus have low curative potential and high relapse rates. Here, we demonstrate that human HSCs can be targeted and eliminated by monoclonal antibodies (mAbs) that bind cell-surface CD117 (c-Kit). We show that an anti-human CD117 mAb, SR-1, inhibits normal cord blood and bone marrow HSCs in vitro. Furthermore, SR-1 and clinical-grade humanized anti-human CD117 mAb, AMG 191, deplete normal and MDS HSCs in vivo in xenograft mouse models. Anti-CD117 mAbs also facilitate the engraftment of normal donor human HSCs in MDS xenograft mouse models, restoring normal human hematopoiesis and eradicating aggressive pathologic MDS cells. This study is the first to demonstrate that anti-human CD117 mAbs have potential as novel therapeutics to eradicate MDS HSCs and augment the curative effect of allogeneic HCT for this disease. Moreover, we establish the foundation for use of these antibody agents not only in the treatment of MDS but also for the multitude of other HSC-driven blood and immune disorders for which transplant can be disease-altering.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: I.L.W. and A.C. are inventors on patents that include the use of anti-CD117 antibodies in HCT conditioning. I.L.W. and J.A.S. are inventors on patents that pair anti-CD47 agents and anti-CD117 antibodies for HCT conditioning. I.L.W. is a cofounder, stockholder, and Director of Forty Seven, Inc, which has licensed these patents from Stanford University. J.A.S. has equity ownership in Forty Seven, Inc. A.C. has equity ownership in Forty Seven, Inc; Magenta Therapeutics; Beam Therapeutics; Editas Medicines; and Global Blood Therapeutics. The remaining authors declare no competing financial interests.
Figures

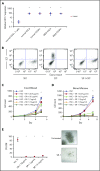

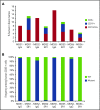
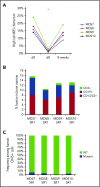
Comment in
-
Selective CD117+ HSC exchange therapy.Blood. 2019 May 9;133(19):2007-2009. doi: 10.1182/blood-2019-03-900894. Blood. 2019. PMID: 31072963 No abstract available.
References
-
- Sutton L, Chastang C, Ribaud P, et al. Factors influencing outcome in de novo myelodysplastic syndromes treated by allogeneic bone marrow transplantation: a long-term study of 71 patients Société Française de Greffe de Moelle. Blood. 1996;88(1):358-365. - PubMed
-
- de Witte T, Hermans J, Vossen J, et al. Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000;110(3):620-630. - PubMed
-
- Sierra J, Pérez WS, Rozman C, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997-2004. - PubMed
-
- Nevill TJ, Fung HC, Shepherd JD, et al. Cytogenetic abnormalities in primary myelodysplastic syndrome are highly predictive of outcome after allogeneic bone marrow transplantation. Blood. 1998;92(6):1910-1917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

