In Vitro Evaluation of the Drug Interaction Potential of Doravirine
- PMID: 30745395
- PMCID: PMC6437506
- DOI: 10.1128/AAC.02492-18
In Vitro Evaluation of the Drug Interaction Potential of Doravirine
Abstract
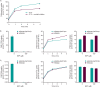
Doravirine is a novel nonnucleoside reverse transcriptase inhibitor for the treatment of human immunodeficiency virus type 1 infection. In vitro studies were conducted to assess the potential for drug interactions with doravirine via major drug-metabolizing enzymes and transporters. Kinetic studies confirmed that cytochrome P450 3A (CYP3A) plays a major role in the metabolism of doravirine, with ∼20-fold-higher catalytic efficiency for CYP3A4 versus CYP3A5. Doravirine was not a substrate of breast cancer resistance protein (BCRP) and likely not a substrate of organic anion transporting polypeptide 1B1 (OATP1B1) or OATP1B3. Doravirine was not a reversible inhibitor of major CYP enzymes (CYP1A2, -2B6, -2C8, -2C9, -2C19, -2D6, and -3A4) or of UGT1A1, nor was it a time-dependent inhibitor of CYP3A4. No induction of CYP1A2 or -2B6 was observed in cultured human hepatocytes; small increases in CYP3A4 mRNA (≤20%) were reported at doravirine concentrations of ≥10 μM but with no corresponding increase in enzyme activity. In vitro transport studies indicated a low potential for interactions with substrates of BCRP, P-glycoprotein, OATP1B1 and OATP1B3, the bile salt extrusion pump (BSEP), organic anion transporter 1 (OAT1) and OAT3, organic cation transporter 2 (OCT2), and multidrug and toxin extrusion 1 (MATE1) and MATE2K proteins. In summary, these in vitro findings indicate that CYP3A4 and CYP3A5 mediate the metabolism of doravirine, although with different catalytic efficiencies. Clinical trials reported elsewhere confirm that doravirine is subject to drug-drug interactions (DDIs) via CYP3A inhibitors and inducers, but they support the notion that DDIs (either direction) are unlikely via other major drug-metabolizing enzymes and transporters.
Keywords: HIV; doravirine; drug interactions.
Copyright © 2019 American Society for Microbiology.
Figures



References
-
- Marcus JL, Chao CR, Leyden WA, Xu L, Quesenberry CP Jr, Klein DB, Towner WJ, Horberg MA, Silverberg MJ. 2016. Narrowing the gap in life expectancy between HIV-infected and HIV-uninfected individuals with access to care. J Acquir Immune Defic Syndr 73:39–46. doi:10.1097/QAI.0000000000001014. - DOI - PMC - PubMed
-
- Merck & Co., Inc. 2018. PIFELTRO (doravirine) prescribing information. https://www.merck.com/product/usa/pi_circulars/p/pifeltro/pifeltro_pi.pdf.
-
- Merck & Co., Inc. 2018. DELSTRIGO (doravirine, lamivudine, and tenofovir disoproxil fumarate) prescribing information. https://www.merck.com/product/usa/pi_circulars/d/delstrigo/delstrigo_pi.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

