Anti-tumorigenic and Platinum-Sensitizing Effects of Apolipoprotein A1 and Apolipoprotein A1 Mimetic Peptides in Ovarian Cancer
- PMID: 30745873
- PMCID: PMC6360149
- DOI: 10.3389/fphar.2018.01524
Anti-tumorigenic and Platinum-Sensitizing Effects of Apolipoprotein A1 and Apolipoprotein A1 Mimetic Peptides in Ovarian Cancer
Abstract
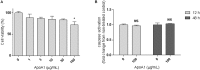
Objective: Apolipoprotein A1 (ApoA1) is remarkably decreased in serum and ovarian tissues of ovarian cancer patients. ApoA1 and ApoA1 mimetic peptides can sequestrate pro-inflammatory phospholipids, some of which are known to activate a variety of oncogenic pathways. Besides, more intrinsic anti-tumorigenic properties, independent from interaction with lipids, have also been described for ApoA1. We aimed to disclose the effects of ApoA1 and a mimetic peptide on the malignant phenotype of ovarian cancer cells, particularly regarding cell viability, invasiveness and platinum sensitization. Methods: Cells viability was assessed by MTS assay. Extracellular matrix invasion was assessed by transwell and spheroid invasion assays. Western blotting was performed to evaluate the effect of test compounds on intracellular pathways. Sensitization assays were performed in vitro and in the biologically relevant in ovo chorioallantoic membrane model. Results: Both ApoA1 and the mimetic peptide, at a concentration of 100 μg/mL, were able to decrease the viability of SKOV3, CAOV3, and OVCAR3 cells (p < 0.05). The peptide at this concentration was not able to affect the viability of immortalized non-neoplastic ovarian cells (p > 0.05). ApoA1 decreased SKOV3 cells invasiveness at 300 μg/mL after 72 and 96 h of exposure (p < 0.05), while the ApoA1 mimetic peptide prevented cell invasion at 50 and 100 μg/mL (p < 0.01). Treatment with 100 μg/mL of ApoA1 mimetic peptide decreased Akt phosphorylation in SKOV3 cells (p < 0.01). Accordingly, treatment with increasing concentrations of the peptide sensitized SKOV3, OVCAR3 and CAOV3 cells to cisplatin. This synergistic effect was observed both in vitro and in ovo. Conclusions: These results support the role of ApoA1 and ApoA1 mimetics as suppressors of ovarian tumorigenesis and as chemo-sensitising agents.
Keywords: ApoA1 mimetic peptides; apolipoprotein A1; invasiveness; ovarian cancer; platinum sensitization.
Figures






References
-
- Cheraghchi-Bashi A., Parker C. A., Curry E., Salazar J.-F., Gungor H., Saleem A., et al. (2015). A putative biomarker signature for clinically effective AKT inhibition: correlation of in vitro, in vivo and clinical data identifies the importance of modulation of the mTORC1 pathway. Oncotarget 6 41736–41749. 10.18632/oncotarget.6153 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

