Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease
- PMID: 30746140
- PMCID: PMC6366145
- DOI: 10.1093/ckj/sfy013
Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants with chronic kidney disease
Abstract
Background: This study assessed the short-term safety and efficacy of daprodustat (an oral hypoxia-inducible factor-prolyl hydroxylase inhibitor) to achieve a target hemoglobin in patients with anemia of chronic kidney disease (CKD).
Methods: Patients (n = 252) with Stages 3-5 CKD not receiving dialysis were enrolled in this 24-week, multicenter trial [hemoglobin entry criteria: 8-10 g/dL (Cohort 1) or 8-11 g/dL (Cohort 2) for recombinant human erythropoietin (rhEPO)-naïve participants; 9-10.5 g/dL (Cohort 1) or 9-11.5 g/dL (Cohort 2) for rhEPO users]. rhEPO-naïve participants were randomized 3:1 to daprodustat (1, 2 or 4 mg) or control (rhEPO per standard of care). rhEPO users were randomized 1:1 to daprodustat 2 mg or control. Study medication was titrated to maintain hemoglobin 9-10.5 g/dL (Cohort 1) or 10-11.5 g/dL (Cohort 2). Hemoglobin, iron metabolism markers and safety parameters were measured every 4 weeks.
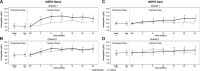
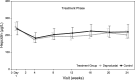
Results: Mean hemoglobin levels at Week 24 were 10.2 g/dL (Cohort 1) and 10.9 g/dL (Cohort 2) in the daprodustat group and 10.7 g/dL (Cohort 1) and 11.0 g/dL (Cohort 2) in the control group. Participants had hemoglobin levels within the target range a median of 82% and 66% of the time between Weeks 12 and 24 in the daprodustat and control groups, respectively. The adverse event profile was consistent with clinical events in the CKD population.
Conclusions: Daprodustat effectively maintained target hemoglobin over 24 weeks in CKD patients with anemia who were rhEPO naïve or had switched from existing rhEPO therapy.
Keywords: chronic kidney disease; daprodustat; hypoxia-inducible factor; prolyl hydroxylase inhibitor; recombinant human erythropoietin.
Figures

References
-
- Bonomini M, Del Vecchio L, Sirolli V. et al. New treatment approaches for the anemia of CKD. Am J Kidney Dis 2016; 67: 133–142 - PubMed
-
- Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 - PubMed
-
- Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 - PubMed
-
- Pfeffer MA, Burdmann EA, Chen C-Y. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 - PubMed
-
- Jenkins J. Statement regarding erythropoiesis-stimulating agents (ESA) before the Committee on Ways and Means Subcommittee on Health, US House of Respresentatives. Government Publishing Office https://www.gpo.gov/fdsys/pkg/CHRG-110hhrg49981/pdf/CHRG-110hhrg49981.pdf. Published 26 June 2007 (20 December 2017, date last accessed)
LinkOut - more resources
Full Text Sources
Other Literature Sources

