Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis
- PMID: 30746141
- PMCID: PMC6366140
- DOI: 10.1093/ckj/sfy014
Daprodustat for anemia: a 24-week, open-label, randomized controlled trial in participants on hemodialysis
Abstract
Background: This study evaluated the hemoglobin dose response, other efficacy measures and safety of daprodustat, an orally administered, hypoxia-inducible factor prolyl hydroxylase inhibitor in development for anemia of chronic kidney disease.
Methods: Participants (n = 216) with baseline hemoglobin levels of 9-11.5 g/dL on hemodialysis (HD) previously receiving stable doses of recombinant human erythropoietin (rhEPO) were randomized in a 24-week dose-range, efficacy and safety study. Participants discontinued rhEPO and then were randomized to receive daily daprodustat (4, 6, 8, 10 or 12 mg) or control (placebo for 4 weeks then open-label rhEPO as required). After 4 weeks, doses were titrated to achieve a hemoglobin target of 10-11.5 g/dL. The primary outcome was characterization of the dose-response relationship between daprodustat and hemoglobin at 4 weeks; additionally, the efficacy and safety of daprodustat were assessed over 24 weeks.
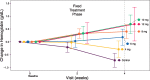
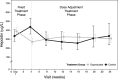
Results: Over the first 4 weeks, the mean hemoglobin change from baseline increased dose-dependently from -0.29 (daprodustat 4 mg) to 0.69 g/dL (daprodustat 10 and 12 mg). The mean change from baseline hemoglobin (10.4 g/dL) at 24 weeks was 0.03 and -0.11 g/dL for the combined daprodustat and control groups, respectively. The median maximum observed plasma EPO levels in the control group were ∼14-fold higher than in the combined daprodustat group. Daprodustat demonstrated an adverse event profile consistent with the HD population.
Conclusions: Daprodustat produced dose-dependent changes in hemoglobin over the first 4 weeks after switching from a stable dose of rhEPO as well as maintained hemoglobin target levels over 24 weeks.
Keywords: anemia; chronic kidney disease; daprodustat; hemodialysis; recombinant human erythropoietin.
Figures


References
-
- United States Renal Data System. 2013 Atlas of CKD and ESRD. Clinical indicators and preventive care https://www.usrds.org/2013/pdf/v2_ch2_13.pdf (3 February 2017, date last accessed)
-
- Locatelli F, Del Vecchio L.. New strategies for anaemia management in chronic kidney disease. Contrib Nephrol 2017; 189: 184–188 - PubMed
-
- Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 - PubMed
-
- Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

