N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication
- PMID: 30746681
- PMCID: PMC6370586
- DOI: 10.1002/14651858.CD012357.pub2
N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication
Abstract
Background: Helicobacter pylori (H pylori) is one of the most common pathogens to establish and cause infection in human beings, affecting about 50% of the world's population. Prevalence may be as high as 83% in Latin American countries and as low as 17% in North America. Approximately 20% of infected people will manifest disease; people at high risk include those who live in low- and middle-income countries with poor sanitary conditions, since the mechanism of transmission seems to be oral-oral or faecal-oral (mostly during infancy). There are several antibiotic regimens to treat the infection, but antibiotic resistance is growing around the world. New adjuvant drugs - such as probiotics, statins, curcumin, and N-acetylcysteine (NAC) - are being tested to enhance eradication rates.N-acetylcysteine can destabilise the biofilm structure; it also has synergic action with antibiotics, and bactericidal effects. In addition, NAC has antioxidant properties, and has a primary mucolytic effect by reducing the thickness of the gastric mucus layer, both of which may exert beneficial adjuvant effects on H pylori eradication.
Objectives: To assess the efficacy and safety of N-acetylcysteine as an adjuvant therapy to antibiotics for Helicobacter pylori eradication.
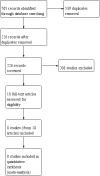
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (1966 to April 2018), Embase (1988 to April 2018), CINAHL (1982 to April 2018), LILACS (1982 to April 2018), grey literature databases and trials registries. We handsearched the reference lists of relevant studies. We screened 726 articles and assessed 18 for eligibility.
Selection criteria: We included randomised controlled trials (RCTs) of any antibiotic regimen plus NAC, in adults infected with H pylori. To be included, trials had to use a control consisting of the same antibiotic regimen with or without placebo. Outcomes of interest were eradication rates, and gastrointestinal, toxic, and allergic adverse events. Reporting of the primary outcomes listed here was not an inclusion criterion for the review.
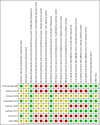
Data collection and analysis: Two review authors independently reviewed and extracted data and completed the 'Risk of bias' assessments. A third review author independently confirmed the 'Risk of bias' assessments. We used Review Manager 5 software for data analysis. We contacted study authors if there was missing information.
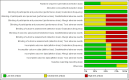
Main results: We included eight RCTs (with a total of 559 participants) in this review. The studies recruited outpatients aged between 17 and 76 years who were referred to endoscopy centres in several different countries. The certainty of evidence was reduced for most outcomes due to the poor methodological quality of included studies; issues mainly related to the generation of allocation sequence, allocation concealment, and blinding (this last domain related specifically to adverse outcomes).We are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates, compared with the addition of placebo or no NAC (38.8% versus 49.1%, risk ratio (RR) 0.74, 95% confidence interval (CI) 0.51 to 1.08; participants = 559; studies = eight; very low-certainty evidence). A post-hoc sensitivity analysis, in which we removed studies that tested antibiotic regimens no longer recommended in clinical practice, showed that the addition of NAC may improve eradication rates compared to control (27.2% versus 37.6%, RR 0.71, 95% CI 0.53 to 0.94; participants = 397; published studies = five).We are uncertain whether NAC is associated with a higher risk of gastrointestinal adverse events compared to control (23.9% versus 18.9%, RR 1.25, 95% CI 0.85 to 1.85; participants = 336; studies = five; very low-certaintyevidence), or allergic adverse events (2% versus 0%, RR 2.98, 95% CI 0.32 to 27.74; participants = 336; studies = five; very low-certainty evidence). There were no reports of toxic adverse events amongst included studies.
Authors' conclusions: We are uncertain whether the addition of NAC to antibiotics improves H pylori eradication rates compared with the addition of placebo or no NAC. Due to the clinical, statistical and methodological heterogeneity found in included studies, and the uncertainty observed when analysing therapy subgroups, any possible beneficial effect of NAC should be regarded cautiously.We are uncertain whether NAC is associated with a higher risk of gastrointestinal or allergic adverse events compared with placebo or no NAC. There were no reports of toxic adverse events amongst the included studies.Further large, well-designed, randomised clinical studies should be conducted, with good reporting standards and appropriate collection of efficacy and safety outcomes, especially for current recommended antibiotic regimens.
Conflict of interest statement
None of the review authors are sponsored, employed, or involved in clinical studies of antimicrobial
LEF: none known.
CB: none known.
ALM: none known.
CGZ: none known.
RR: none known.
Figures







Update of
References
References to studies included in this review
Cammarota 2009 {published data only}
-
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clinical Gastroenterology and Hepatology 2010;8(9):817‐20.e3. - PubMed
-
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Histopathologic and morphologic evaluations of resistant strains of H. pylori, and consequent new therapeutic approaches: usefulness of N‐acetil‐cysteine and culture‐guided antibiotics. Helicobacter 2009;14(4):334.
-
- Cammarota G, Ianiro G, Cazzato A, Piscaglia AC, Lauritano C, Gigante G, et al. Pre‐treatment with N‐acetil‐cysteine and culture‐guided rescue therapy for eradication of Helicobacter pylori infection. Gastroenterology 2009;136(5 Suppl 1):A345W.
Emami 2014 {published data only}
Gurbuz 2005 {published data only}
-
- Gurbuz AK, Ozel AM, Ozturk R, Yildirim S, Yazgan Y, Demirturk L. Effect of N‐acetyl cysteine onHelicobacter pylori. Southern Medical Journal 2005;98(11):1095‐7. - PubMed
Hamidian 2015 {published data only}
Hansen 1994 {published data only}
-
- Hansen IM, Larsen JF, Qvist N, Lundborg CJ, Brandslund I, Axelsson CK. N‐acetylcysteine does not enhance eradication of Helicobacter pylori by amoxycillin. European Journal of Clinical Research 1994;5:185‐91.
Karbasi 2013 {published data only}
-
- Karbasi A, Hossein HS, Shohrati M, Amini M, Najafian B. Effect of oral N‐acetyl cysteine on eradication of Helicobacter pylori in patients with dyspepsia. Minerva Gastroenterologica e Dietologica 2013;59(1):107‐12. - PubMed
Yoon 2014 {published data only}
-
- Yoon H, Lee DH, Jang ES, Kim J, Shin CM, Park YS, et al. The effects of N‐acetylcysteine on first‐line 10‐day sequential therapy for Helicobacter pylori infection: A randomized controlled trial. United European Gastroenterology Journal 2014;2(1 Suppl 1):A4301.
Zala 1994 {published data only}
-
- Zala G, Flury R, Wust J, Meyenberger C, Ammann R, Wirth HP. N‐acetylcysteine improves eradication of Helicobacter pylori by omeprazole/amoxicillin in cigarette smokers. Schweizerische Medizinische Wochenschrift 1994;124(31‐32):1391‐7. - PubMed
References to ongoing studies
NCT01572597 {published data only}
-
- NCT01572597. Increased re‐eradication rate of Helicobacter pylori by adding N‐acetylcysteine or metronidazole to the triple therapy [Increased second‐line eradication rate of Helicobacter pylori by adding N‐acetylcysteine or metronidazole to the conventional triple therapy]. clinicaltrials.gov/ct2/show/NCT01572597 (first received 6 April 2012).
NCT02249546 {published data only}
-
- NCT02249546. Efficacy of acetylcysteine‐containing triple therapy in the first line of Helicobacter pylori infection [Comparison of the efficacy of triple therapy with or without acetylcysteine in the first line of Helicobacter pylori infection ‐ a multicenter randomized comparative trial]. clinicaltrials.gov/ct2/show/NCT02249546 (first received 25 September 2014).
Additional references
Aslam 2007
Aslam 2011
Blaser 2005
-
- Blaser MJ. An endangered species in the stomach. Scientific American 2005;292(2):38‐45. [PUBMED: 15715390] - PubMed
Borgstrom 1986
-
- Borgstrom L, Kagedal B, Paulsen O. Pharmacokinetics of N‐acetylcysteine in man. European Journal of Clinical Pharmacology 1986;31(2):217‐22. [PUBMED: 3803419] - PubMed
Calvet 2013
-
- Calvet X, Ramirez Lazaro MJ, Lehours P, Megraud F. Diagnosis and epidemiology of Helicobacter pylori infection. Helicobacter 2013;18 Suppl 1:5‐11. [PUBMED: 24011238] - PubMed
Cammarota 2010
-
- Cammarota G, Branca G, Ardito F, Sanguinetti M, Ianiro G, Cianci R, et al. Biofilm demolition and antibiotic treatment to eradicate resistant Helicobacter pylori: a clinical trial. Clinical Gastroenterology and Hepatology 2010;8(9):817‐20.e3. [PUBMED: 20478402] - PubMed
Cammarota 2012
-
- Cammarota G, Sanguinetti M, Gallo A, Posteraro B. Review article: biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Alimentary pharmacology & therapeutics 2012;36(3):222‐30. [PUBMED: 22650647] - PubMed
Carron 2006
-
- Carron MA, Tran VR, Sugawa C, Coticchia JM. Identification of Helicobacter pylori biofilms in human gastric mucosa. Journal of Gastrointestinal Surgery 2006;10(5):712‐7. [PUBMED: 16713544] - PubMed
Chaabane 2011
-
- Chaabane NB, Mansour IB, Hellara O, Loghmeri H, Bdioui F, Safer L, et al. Role of Helicobacter pylori infection in iron deficiency anemia [Role de l'infection par l'Helicobacter pylori dans l'anemie ferriprive]. Presse Medicale 2011;40(3):239‐47. [PUBMED: 21196096] - PubMed
Correa 1992
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process ‐ First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Research 1992;52(24):6735‐40. [PUBMED: 1458460] - PubMed
Coticchia 2006
-
- Coticchia JM, Sugawa C, Tran VR, Gurrola J, Kowalski E, Carron MA. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer disease. Journal of Gastrointestinal Surgery 2006;10(6):883‐9. [PUBMED: 16769546] - PubMed
De Caro 1989
-
- Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittel‐Forschung 1989;39(3):382‐6. [PUBMED: 2757663] - PubMed
Dunn 1997
EHSG 2012
-
- European Helicobacter Study Group (EHSG). Management of Helicobacter pylori infection—the Maastricht IV/ Florence Consensus Report. GUT 2012;61(5):302‐84. - PubMed
ERC 2014
-
- Cochrane Editorial Resources Committee (ERC). ERC data collection form for intervention review for RCTs only. www.community‐archive.cochrane.org/sites/default/files/uploads/forums/u389/ERC%20data%... (accessed 12 September 2016).
Ermis 2015
Fair 2014
Fontes 2016
Garcia 2014
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 9 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Graham 2010
-
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59(8):1143‐53. [PUBMED: 20525969] - PubMed
Guyatt 2006
-
- Guyatt G, Gutterman D, Baumann MH, Addrizzo‐Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest 2006;129(1):174‐81. [PUBMED: 16424429] - PubMed
Hall‐Stoodley 2009
-
- Hall‐Stoodley L, Stoodley P. Evolving concepts in biofilm infections. Cellular Microbiology 2009;11(7):1034‐43. [PUBMED: 19374653] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Altman DG, Sterne JAC, Cochrane Statistical Methods Group, Cochrane Bias Methods Group. Chapter 8.5: The Cochrane Collaboration's tool for assessing risk of bias. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunt 2011
-
- Hunt RH, Xiao SD, Megraud F, Leon‐Barua R, Bazzoli F, Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. Journal of Gastrointestinal and Liver Diseases 2011;20(3):299‐304. [PUBMED: 21961099] - PubMed
Itskoviz 2017
-
- Itskoviz D, Boltin D, Leibovitzh H, Tsadok Perets T, Comaneshter D, Cohen A, et al. Smoking increases the likelihood of Helicobacter pylori treatment failure. Digestive and Liver Disease 2017;49(7):764‐8. [PUBMED: 28427781] - PubMed
Kao 2016
Kelly 1998
-
- Kelly GS. Clinical applications of N‐acetylcysteine. Alternative Medicine Review 1998;3(2):114‐27. [PUBMED: 9577247] - PubMed
Kuipers 1997
-
- Kuipers EJ. Helicobacter pylori and the risk and management of associated diseases: gastritis, ulcer disease, atrophic gastritis and gastric cancer. Alimentary Pharmacology & Therapeutics 1997;11 Suppl 1:71‐88. [PUBMED: 9146793] - PubMed
Malfertheiner 2009
-
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet 2009;374(9699):1449‐61. [PUBMED: 19683340] - PubMed
Malfertheiner 2012
-
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection — the Maastricht IV/ Florence Consensus Report. Gut 2012;61(5):646‐64. [PUBMED: 22491499] - PubMed
Malfertheiner 2017
-
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection‐the Maastricht V/Florence Consensus Report. Gut 2017;66(1):6‐30. [PUBMED: 27707777] - PubMed
Marshall 1984
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1(8390):1311‐5. [PUBMED: 6145023] - PubMed
Millea 2009
-
- Millea PJ. N‐acetylcysteine: multiple clinical applications. American Family Physician 2009;80(3):265‐9. [PUBMED: 19621836] - PubMed
Moher 2009
Momtaz 2012
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sherwood 2002
Stasi 2009
-
- Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009;113(6):1231‐40. [PUBMED: 18945961] - PubMed
Venerito 2013
-
- Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta‐analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion 2013;88(1):33‐45. [PUBMED: 23880479] - PubMed
Yonezawa 2010
-
- Yonezawa H, Osaki T, Kurata S, Zaman C, Hanawa T, Kamiya S. Assessment of in vitro biofilm formation by Helicobacter pylori. Journal of Gastroenterology and Hepatology 2010;25 Suppl 1:S90‐4. [PUBMED: 20586874] - PubMed
Yoon 2015
Zhao 2014
-
- Zhao B, Zhao J, Cheng WF, Shi WJ, Liu W, Pan XL, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta‐analysis of randomized controlled studies with 12‐month follow‐up. Journal of Clinical Gastroenterology 2014;48(3):241‐7. [PUBMED: 24002127] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

