HnRNPL inhibits the osteogenic differentiation of PDLCs stimulated by SrCl2 through repressing Setd2
- PMID: 30746871
- PMCID: PMC6433863
- DOI: 10.1111/jcmm.14166
HnRNPL inhibits the osteogenic differentiation of PDLCs stimulated by SrCl2 through repressing Setd2
Abstract
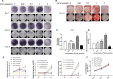
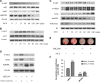
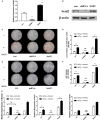
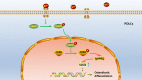
Osteoporosis has been shown to intensify bone loss caused by periodontitis and both share common risk factors. One strategy utilized to manage the disease has been via the release of Sr ions by Strontium Ranelate having a direct effect on preventing osteoclast activation and promoting osteoblast differentiation. Previously we have developed and characterized porous Sr-mesoporous bioactive glass (Sr-MBG) scaffolds and demonstrated their ability to promote periodontal regeneration when compared to MBG alone. Our group further discovered a splicing factor, heterogeneous nuclear ribonucleoprotein L (hnRNPL), was drastically down-regulated in periodontal ligament stem cells (PDLCs) stimulated by Sr through the activation of AKT pathway. Furthermore, hnRNPL restrained the osteogenic differentiation of PDLCs through down-regulating H3K36me3-specific methyltransferase Setd2. The goal of the present study was to investigate the mechanism of periodontal regeneration stimulated by Sr It was first found that the epigenetic mechanism of splicing factor hnRNPL participated in the osteogenesis processing of PDLCs stimulated by SrCl2 . Meanwhile, the different role of hnRNPL and SET domain containing 2 (Setd2) may provide some implication of the treatment of periodontitis patients simultaneously suffering from osteoporosis.
Keywords: RNA splicing; bioengineering; hnRNPL; osteoporosis; periodontal ligament stem cells.
© 2019 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures


Similar articles
-
Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering.Acta Biomater. 2012 Oct;8(10):3805-15. doi: 10.1016/j.actbio.2012.06.023. Epub 2012 Jun 28. Acta Biomater. 2012. PMID: 22750735
-
Setd2 is associated with strontium-induced bone regeneration.Acta Biomater. 2017 Apr 15;53:495-505. doi: 10.1016/j.actbio.2017.02.025. Epub 2017 Feb 20. Acta Biomater. 2017. PMID: 28219807
-
Setd7 and its contribution to Boron-induced bone regeneration in Boron-mesoporous bioactive glass scaffolds.Acta Biomater. 2018 Jun;73:522-530. doi: 10.1016/j.actbio.2018.04.033. Epub 2018 Apr 20. Acta Biomater. 2018. PMID: 29684621
-
Epigenetic regulation of osteogenic differentiation of periodontal ligament stem cells in periodontitis.Oral Dis. 2023 Oct;29(7):2529-2537. doi: 10.1111/odi.14491. Epub 2023 Jan 13. Oral Dis. 2023. PMID: 36582112 Review.
-
Wnt signaling: An attractive target for periodontitis treatment.Biomed Pharmacother. 2021 Jan;133:110935. doi: 10.1016/j.biopha.2020.110935. Epub 2020 Nov 20. Biomed Pharmacother. 2021. PMID: 33227711 Review.
Cited by
-
Epigenetic Regulation of Methylation in Determining the Fate of Dental Mesenchymal Stem Cells.Stem Cells Int. 2022 Sep 22;2022:5015856. doi: 10.1155/2022/5015856. eCollection 2022. Stem Cells Int. 2022. PMID: 36187229 Free PMC article. Review.
-
Long non-coding RNA lincRNA-erythroid prosurvival (EPS) alleviates cerebral ischemia/reperfusion injury by maintaining high-temperature requirement protein A1 (Htra1) stability through recruiting heterogeneous nuclear ribonucleoprotein L (HNRNPL).Bioengineered. 2022 May;13(5):12248-12260. doi: 10.1080/21655979.2022.2074738. Bioengineered. 2022. PMID: 35549989 Free PMC article.
-
Identification of biomarkers associated with diagnosis of postmenopausal osteoporosis patients based on bioinformatics and machine learning.Front Genet. 2023 Jul 3;14:1198417. doi: 10.3389/fgene.2023.1198417. eCollection 2023. Front Genet. 2023. PMID: 37465165 Free PMC article.
-
The Role of Epigenetic in Dental and Oral Regenerative Medicine by Different Types of Dental Stem Cells: A Comprehensive Overview.Stem Cells Int. 2022 Jun 9;2022:5304860. doi: 10.1155/2022/5304860. eCollection 2022. Stem Cells Int. 2022. PMID: 35721599 Free PMC article. Review.
-
Applications of Bioactive Strontium Compounds in Dentistry.J Funct Biomater. 2024 Jul 31;15(8):216. doi: 10.3390/jfb15080216. J Funct Biomater. 2024. PMID: 39194654 Free PMC article. Review.
References
-
- Takuma T, Oishi K, Manabe T, Yoneda S, Nagata T. Buccal bone resorption around posterior implants after surgery: a 1‐year prospective study. Int J Oral Max Impl. 2014;29:634‐641. - PubMed
-
- Yu BH, Zhou Q, Wang ZL. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio‐Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J Biomater Appl. 2014;29:243‐253. - PubMed
-
- Penoni DC, Torres SR, Farias ML, Fernandes TM, Luiz RR, Leao AT. Association of osteoporosis and bone medication with the periodontal condition in elderly women. Osteoporos Int. 2016;27:1887‐1896. - PubMed
-
- Hou KL, Lin SK, Kok SH, et al. Increased expression of glutaminase in osteoblasts promotes macrophage recruitment in periapical lesions. J Endod. 2017;43:602‐608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

