Mitochondrial integrity in neurodegeneration
- PMID: 30746905
- PMCID: PMC6566061
- DOI: 10.1111/cns.13105
Mitochondrial integrity in neurodegeneration
Abstract
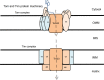
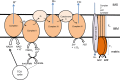
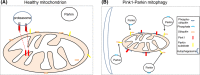
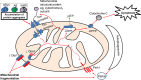
The mitochondrion is a unique organelle with a diverse range of functions. Mitochondrial dysfunction is a key pathological process in several neurodegenerative diseases. Mitochondria are mostly important for energy production; however, they also have roles in Ca2+ homeostasis, ROS production, and apoptosis. There are two major systems in place, which regulate mitochondrial integrity, mitochondrial dynamics, and mitophagy. These two processes remove damaged mitochondria from cells and protect the functional mitochondrial population. These quality control systems often become dysfunctional during neurodegenerative diseases, such as Parkinson's and Alzheimer's disease, causing mitochondrial dysfunction and severe neurological symptoms.
Keywords: cytotoxicity; mitochondrion; mitophagy; neurodegeneration.
© 2019 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Veltri KL, Espiritu M, Singh G. Distinct genomic copy number in mitochondria of different mammalian organs. J Cell Physiol. 1990;143(1):160‐164. - PubMed
-
- Bertram R, Gram Pedersen M, Luciani DS, Sherman A. A simplified model for mitochondrial ATP production. J Theor Biol. 2006;243(4):575‐586. - PubMed
-
- Gunter TE, Buntinas L, Sparagna GC, Gunter KK. The Ca2+ transport mechanisms of mitochondria and Ca2+ uptake from physiological‐type Ca2+ transients. Biochim Biophys Acta. 1998;1366(1‐2):5‐15. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

