Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial
- PMID: 30747501
- PMCID: PMC6618246
- DOI: 10.1002/art.40851
Etanercept and Methotrexate as Monotherapy or in Combination for Psoriatic Arthritis: Primary Results From a Randomized, Controlled Phase III Trial
Abstract
Objective: To examine the efficacy of methotrexate monotherapy relative to etanercept monotherapy and the value of combining methotrexate and etanercept for the treatment of patients with psoriatic arthritis (PsA).
Methods: In this double-blind study, 851 patients with PsA were randomized to 1 of 3 treatment arms, as follows: oral methotrexate (20 mg) plus subcutaneous placebo given weekly (n = 284), subcutaneous etanercept (50 mg) plus oral placebo given weekly (n = 284), or subcutaneous etanercept (50 mg) plus oral methotrexate (20 mg) given weekly (combination therapy; n = 283). The American College of Rheumatology 20% improvement (ACR20) response and Minimal Disease Activity (MDA) response at week 24 were the primary end point and key secondary end point, respectively. Other measures of inflammatory arthritis, radiographic progression, and nonarticular disease manifestations were also assessed.
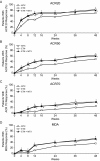
Results: Patients with PsA had a mean ± SD age of 48.4 ± 13.1 years, and the mean ± SD duration of PsA was 3.2 ± 6.3 years (median 0.6 years). ACR20 and MDA response rates at week 24 were significantly greater in patients who received etanercept monotherapy compared with those who received methotrexate monotherapy (ACR20, 60.9% versus 50.7% of patients [P = 0.029]; MDA, 35.9% versus 22.9% of patients [P = 0.005]), and both were significantly greater in the combination therapy group compared with the methotrexate monotherapy group at week 24 (ACR20, 65.0% versus 50.7% of patients [P = 0.005]; MDA, 35.7% versus 22.9% of patients [P = 0.005]). Other secondary outcomes (ACR50 and ACR70 response rates, proportions of patients achieving a Very Low Disease Activity score, and PsA disease activity scores) showed between-group differences that were consistent with the primary and key secondary end point results. Furthermore, patients in both etanercept treatment arms showed less radiographic progression at week 48 compared with patients who received methotrexate monotherapy. Outcomes were similar in the combination therapy and etanercept monotherapy groups, except for some skin end points. No new safety signals were seen.
Conclusion: Etanercept monotherapy and combination therapy with etanercept and methotrexate showed greater efficacy than methotrexate monotherapy in patients with PsA, according to the ACR and MDA response rates and extent of radiographic progression at follow-up. Overall, combining methotrexate and etanercept did not improve the efficacy of etanercept.
Trial registration: ClinicalTrials.gov NCT02376790.
© 2019 The Authors. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College ofRheumatology.
Figures


Comment in
-
SEAM-PsA: Seems Like Methotrexate Works in Psoriatic Arthritis?Arthritis Rheumatol. 2019 Jul;71(7):1027-1029. doi: 10.1002/art.40872. Arthritis Rheumatol. 2019. PMID: 30816631 Free PMC article. No abstract available.
-
Failure of Etanercept and Methotrexate Combination Therapy to Surpass Etanercept Monotherapy in Psoriatic Arthritis-What About the Joint Counts? Comment on the Article by Mease et al.Arthritis Rheumatol. 2019 Nov;71(11):1965-1966. doi: 10.1002/art.41034. Epub 2019 Sep 20. Arthritis Rheumatol. 2019. PMID: 31271526 No abstract available.
-
Reply.Arthritis Rheumatol. 2020 Jul;72(7):1229-1230. doi: 10.1002/art.41273. Epub 2020 May 28. Arthritis Rheumatol. 2020. PMID: 32266796 No abstract available.
-
Is Methotrexate as Efficacious as Etanercept in Psoriatic Arthritis Patients? Comment on the Article by Mease et al.Arthritis Rheumatol. 2020 Jul;72(7):1227-1229. doi: 10.1002/art.41274. Epub 2020 May 27. Arthritis Rheumatol. 2020. PMID: 32336032 No abstract available.
References
-
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. - PubMed
-
- Eder L, Haddad A, Rosen CF, Lee KA, Chandran V, Cook R, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol 2016;68:915–23. - PubMed
-
- Koo J. Population‐based epidemiologic study of psoriasis with emphasis on quality of life assessment. Dermatol Clin 1996;14:485–96. - PubMed
-
- Biondi Oriente C, Scarpa R, Pucino A, Oriente P. Psoriasis and psoriatic arthritis: dermatological and rheumatological co‐operative clinical report. Acta Derm Venereol Suppl (Stockh) 1989;146:69–71. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

