A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics
- PMID: 30753168
- PMCID: PMC6483000
- DOI: 10.1172/jci.insight.125348
A unique androgen excess signature in idiopathic intracranial hypertension is linked to cerebrospinal fluid dynamics
Abstract
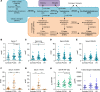
Idiopathic intracranial hypertension (IIH) is a condition of unknown etiology, characterized by elevated intracranial pressure frequently manifesting with chronic headaches and visual loss. Similar to polycystic ovary syndrome (PCOS), IIH predominantly affects obese women of reproductive age. In this study, we comprehensively examined the systemic and cerebrospinal fluid (CSF) androgen metabolome in women with IIH in comparison with sex-, BMI-, and age-matched control groups with either simple obesity or PCOS (i.e., obesity and androgen excess). Women with IIH showed a pattern of androgen excess distinct to that observed in PCOS and simple obesity, with increased serum testosterone and increased CSF testosterone and androstenedione. Human choroid plexus expressed the androgen receptor, alongside the androgen-activating enzyme aldoketoreductase type 1C3. We show that in a rat choroid plexus cell line, testosterone significantly enhanced the activity of Na+/K+-ATPase, a surrogate of CSF secretion. We demonstrate that IIH patients have a unique signature of androgen excess and provide evidence that androgens can modulate CSF secretion via the choroid plexus. These findings implicate androgen excess as a potential causal driver and therapeutic target in IIH.
Keywords: Endocrinology; Neurological disorders; Neuroscience.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

