Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells
- PMID: 30753169
- PMCID: PMC6483008
- DOI: 10.1172/jci.insight.123672
Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells
Abstract
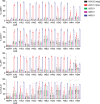
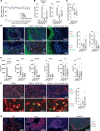
Chimeric antigen receptor (CAR) technology can be used to engineer the antigen specificity of regulatory T cells (Tregs) and improve their potency as an adoptive cell therapy in multiple disease models. As synthetic receptors, CARs carry the risk of immunogenicity, particularly when derived from nonhuman antibodies. Using an HLA-A*02:01-specific CAR (A2-CAR) encoding a single-chain variable fragment (Fv) derived from a mouse antibody, we developed a panel of 20 humanized A2-CARs (hA2-CARs). Systematic testing demonstrated variations in expression, and ability to bind HLA-A*02:01 and stimulate human Treg suppression in vitro. In addition, we developed a new method to comprehensively map the alloantigen specificity of CARs, revealing that humanization reduced HLA-A cross-reactivity. In vivo bioluminescence imaging showed rapid trafficking and persistence of hA2-CAR Tregs in A2-expressing allografts, with eventual migration to draining lymph nodes. Adoptive transfer of hA2-CAR Tregs suppressed HLA-A2+ cell-mediated xenogeneic graft-versus-host disease and diminished rejection of human HLA-A2+ skin allografts. These data provide a platform for systematic development and specificity testing of humanized alloantigen-specific CARs that can be used to engineer specificity and homing of therapeutic Tregs.
Keywords: Immunology; Immunotherapy; Organ transplantation; Tolerance; Transplantation.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

