Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes
- PMID: 30753258
- PMCID: PMC6416106
- DOI: 10.1093/advances/nmy084
Glutamine Metabolism in Macrophages: A Novel Target for Obesity/Type 2 Diabetes
Abstract
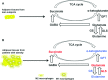
Obesity is a nutritional disorder resulting from a chronic imbalance between energy intake and expenditure. This disease is characterized by inflammation in multiple cell types, including macrophages. M1 macrophage responses are correlated with the progression of obesity or diabetes; therefore, strategies that induce repolarization of macrophages from an M1 to an M2 phenotype may be promising for the prevention of obesity- or diabetes-associated pathology. Glutamine (the most abundant amino acid in the plasma of humans and many other mammals including rats) is effective in inducing polarization of M2 macrophages through the glutamine-UDP-N-acetylglucosamine pathway and α-ketoglutarate produced via glutaminolysis, whereas succinate synthesized via glutamine-dependent anerplerosis or the γ-aminobutyric acid shunt promotes polarization of M1 macrophages. Interestingly, patients with obesity or diabetes show altered glutamine metabolism, including decreases in glutamine and α-ketoglutarate concentrations in serum but increases in succinate concentrations. Thus, manipulation of macrophage polarization through glutamine metabolism may provide a potential target for prevention of obesity- or diabetes-associated pathology.
Keywords: diabetes; glutamine; macrophages; obesity; succinate; α-ketoglutarate.
© 2019 American Society for Nutrition.
Figures


References
-
- Ginhoux F, Schultze JL, Murray PJ, Ochando J, Biswas SK. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17(1):34–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

