Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer
- PMID: 30753272
- PMCID: PMC6503628
- DOI: 10.1093/annonc/mdz018
Overall survival and updated progression-free survival outcomes in a randomized phase II study of combination cediranib and olaparib versus olaparib in relapsed platinum-sensitive ovarian cancer
Abstract
Background: Olaparib is a poly(ADP-ribose) polymerase inhibitor and cediranib is an oral anti-angiogenic. In the primary analysis of this phase II study, combination cediranib/olaparib improved progression-free survival (PFS) compared with olaparib alone in relapsed platinum-sensitive ovarian cancer. This updated analysis was conducted to characterize overall survival (OS) and update PFS outcomes.
Patients and methods: Ninety patients were enrolled to this randomized, open-label, phase II study between October 2011 and June 2013 across nine United States-based academic centers. Data cut-off was 21 December 2016, with a median follow-up of 46 months. Participants had relapsed platinum-sensitive ovarian cancer of high-grade serous or endometrioid histology or had a deleterious germline BRCA1/2 mutation (gBRCAm). Participants were randomized to receive olaparib capsules 400 mg twice daily or cediranib 30 mg daily and olaparib capsules 200 mg twice daily until disease progression.
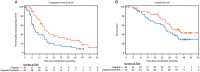
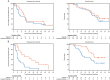
Results: In this updated analysis, median PFS remained significantly longer with cediranib/olaparib compared with olaparib alone (16.5 versus 8.2 months, hazard ratio 0.50; P = 0.007). Subset analyses within stratum defined by BRCA status demonstrated statistically significant improvement in PFS (23.7 versus 5.7 months, P = 0.002) and OS (37.8 versus 23.0 months, P = 0.047) in gBRCA wild-type/unknown patients, although OS was not statistically different in the overall study population (44.2 versus 33.3 months, hazard ratio 0.64; P = 0.11). PFS and OS appeared similar between the two arms in gBRCAm patients. The most common CTCAE grade 3/4 adverse events with cediranib/olaparib remained fatigue, diarrhea, and hypertension.
Conclusions: Combination cediranib/olaparib significantly extends PFS compared with olaparib alone in relapsed platinum-sensitive ovarian cancer. Subset analyses suggest this margin of benefit is driven by PFS prolongation in patients without gBRCAm. OS was also significantly increased by the cediranib/olaparib combination in this subset of patients. Additional studies of this combination are ongoing and should incorporate analyses based upon BRCA status.
Trial registration: Clinicaltrials.gov Identifier NCT0111648.
Keywords: PARP inhibitors; antiangiogenic therapies; combination targeted therapies; ovarian cancer.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Chemotherapy-free treatments: are we ready for prime time?Ann Oncol. 2019 Apr 1;30(4):497-498. doi: 10.1093/annonc/mdz079. Ann Oncol. 2019. PMID: 30835275 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2018. CA Cancer J Clin 2018; 68(1): 7–30. - PubMed
-
- Zejula (niraparib) [package insert]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208447lbl.pdf (29 January 2019, date last accessed).
-
- Zejula (niraparib) [EMA Authorisation]; https://www.ema.europa.eu/en/medicines/human/EPAR/zejula (29 January 2019, date last accessed).
-
- Lynparza (olaparib) [package insert]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208558s001lbl.pdf (29 January 2019, date last accessed).
-
- Rubraca (rucaparib) [package insert]; https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209115s003lbl.pdf (29 January 2019, date last accessed).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

