Motivational Impairment is Accompanied by Corticoaccumbal Dysfunction in the BACHD-Tg5 Rat Model of Huntington's Disease
- PMID: 30753343
- PMCID: PMC7150618
- DOI: 10.1093/cercor/bhz009
Motivational Impairment is Accompanied by Corticoaccumbal Dysfunction in the BACHD-Tg5 Rat Model of Huntington's Disease
Abstract
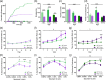
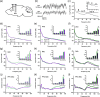
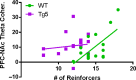
Neuropsychiatric symptoms, such as avolition, apathy, and anhedonia, precede the onset of debilitating motor symptoms in Huntington's disease (HD), and their development may give insight into early disease progression and treatment. However, the neuronal and circuit mechanisms of premanifest HD pathophysiology are not well-understood. Here, using a transgenic rat model expressing the full-length human mutant HD gene, we find early and profound deficits in reward motivation in the absence of gross motor abnormalities. These deficits are accompanied by significant and progressive dysfunction in corticostriatal processing and communication among brain areas critical for reward-driven behavior. Together, our results define early corticostriatal dysfunction as a possible pathogenic contributor to psychiatric disturbances and may help identify potential pharmacotherapeutic targets for the treatment of HD.
Keywords: Huntington’s disease; corticostriatal; motivation; nucleus accumbens; prefrontal cortex.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Compromised Dopaminergic Encoding of Reward Accompanying Suppressed Willingness to Overcome High Effort Costs Is a Prominent Prodromal Characteristic of the Q175 Mouse Model of Huntington's Disease.J Neurosci. 2016 May 4;36(18):4993-5002. doi: 10.1523/JNEUROSCI.0135-16.2016. J Neurosci. 2016. PMID: 27147652 Free PMC article.
-
Lack of mutant huntingtin in cortical efferents improves behavioral inflexibility and corticostriatal dynamics in Huntington's disease mice.J Neurophysiol. 2019 Dec 1;122(6):2621-2629. doi: 10.1152/jn.00777.2018. Epub 2019 Nov 6. J Neurophysiol. 2019. PMID: 31693428 Free PMC article.
-
Downregulation of glial genes involved in synaptic function mitigates Huntington's disease pathogenesis.Elife. 2021 Apr 19;10:e64564. doi: 10.7554/eLife.64564. Elife. 2021. PMID: 33871358 Free PMC article.
-
Disrupted striatal neuron inputs and outputs in Huntington's disease.CNS Neurosci Ther. 2018 Apr;24(4):250-280. doi: 10.1111/cns.12844. CNS Neurosci Ther. 2018. PMID: 29582587 Free PMC article. Review.
-
Corticostriatal network dysfunction in Huntington's disease: Deficits in neural processing, glutamate transport, and ascorbate release.CNS Neurosci Ther. 2018 Apr;24(4):281-291. doi: 10.1111/cns.12828. Epub 2018 Feb 21. CNS Neurosci Ther. 2018. PMID: 29464896 Free PMC article. Review.
Cited by
-
The Role of Hypothalamic Pathology for Non-Motor Features of Huntington's Disease.J Huntingtons Dis. 2019;8(4):375-391. doi: 10.3233/JHD-190372. J Huntingtons Dis. 2019. PMID: 31594240 Free PMC article. Review.
-
Differential Cellular Balance of Olfactory and Vomeronasal Epithelia in a Transgenic BACHD Rat Model of Huntington's Disease.Int J Mol Sci. 2022 Jul 10;23(14):7625. doi: 10.3390/ijms23147625. Int J Mol Sci. 2022. PMID: 35886975 Free PMC article.
References
-
- Abeles M. 1991. Corticonics: Neural Circuits of the Cerebral Cortex [WWW Document]. Camb Core. URL /core/books/corticonics/7BF149062695412A32FFC1255C98B410
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

