Impact of Rotavirus Vaccine Introduction in Children Less Than 2 Years of Age Presenting for Medical Care With Diarrhea in Rural Matlab, Bangladesh
- PMID: 30753368
- PMCID: PMC6880338
- DOI: 10.1093/cid/ciz133
Impact of Rotavirus Vaccine Introduction in Children Less Than 2 Years of Age Presenting for Medical Care With Diarrhea in Rural Matlab, Bangladesh
Abstract
Background: Following the conclusion of a human rotavirus vaccine (HRV) cluster-randomized, controlled trial (CRT) in Matlab, Bangladesh, HRV was included in Matlab's routine immunization program. We describe the population-level impact of programmatic rotavirus vaccination in Bangladesh in children <2 years of age.
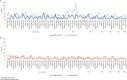
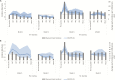
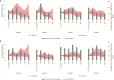
Methods: Interrupted time series were used to estimate the impact of HRV introduction. We used diarrheal surveillance collected between 2000 and 2014 within the 2 service delivery areas (International Centre for Diarrhoeal Disease Research, Bangladesh [icddr,b] service area [ISA] and government service area [GSA]) of the Matlab Health and Demographic Surveillance System, administered by icddr,b. Age group-specific incidence rates were calculated for both rotavirus-positive (RV+) and rotavirus-negative (RV-) diarrhea diagnoses of any severity presenting to the hospital. We used 2 models to assess the impact within each service area: Model 1 used the pre-vaccine time period in all villages (HRV- and control-only) and Model 2 combined the pre-vaccine time period and the CRT time period, using outcomes from control-only villages.
Results: Both models demonstrated a downward trend in RV+ diarrheal incidences in the ISA villages during 3.5 years of routine HRV use, though only Model 2 was statistically significant. Significant impacts of HRV on RV+ diarrhea incidences in GSA villages were not observed in either model. Differences in population-level impacts between the 2 delivery areas may be due to the varied rotavirus vaccine coverage and presentation rates to the hospital.
Conclusions: This study provides initial evidence of the population-level impact of rotavirus vaccines in children <2 years of age in Matlab, Bangladesh. Further studies are needed of the rotavirus vaccine impact after the nationwide introduction in Bangladesh.
Keywords: impact; rotavirus vaccine; time-series.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures

Comment in
-
Rotavirus Vaccines Set to Make Inroads in Asia.Clin Infect Dis. 2019 Nov 27;69(12):2071-2073. doi: 10.1093/cid/ciz137. Clin Infect Dis. 2019. PMID: 30759190 Free PMC article. No abstract available.
Similar articles
-
Effectiveness of a live oral human rotavirus vaccine after programmatic introduction in Bangladesh: A cluster-randomized trial.PLoS Med. 2017 Apr 18;14(4):e1002282. doi: 10.1371/journal.pmed.1002282. eCollection 2017 Apr. PLoS Med. 2017. PMID: 28419095 Free PMC article. Clinical Trial.
-
Impact of vaccination on the risk factors for acute rotavirus diarrhea: An analysis of the data of a cluster randomized trial conducted in a rural area of Bangladesh.Vaccine. 2020 Feb 24;38(9):2190-2197. doi: 10.1016/j.vaccine.2020.01.041. Epub 2020 Jan 23. Vaccine. 2020. PMID: 31983585 Clinical Trial.
-
Rotavirus Vaccine Trials in International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) and Future Use of the Vaccine in Bangladesh.J Infect Dis. 2021 Dec 20;224(12 Suppl 2):S801-S804. doi: 10.1093/infdis/jiab442. J Infect Dis. 2021. PMID: 34528671 Free PMC article.
-
Remaining issues and challenges for rotavirus vaccine in preventing global childhood diarrheal morbidity and mortality.Expert Rev Vaccines. 2012 Feb;11(2):211-20. doi: 10.1586/erv.11.184. Expert Rev Vaccines. 2012. PMID: 22309669 Review.
-
Temporal trends in diarrhea-related hospitalizations and deaths in children under age 5 before and after the introduction of the rotavirus vaccine in four Latin American countries.Vaccine. 2013 Jul 2;31 Suppl 3:C99-108. doi: 10.1016/j.vaccine.2013.05.065. Vaccine. 2013. PMID: 23777700 Review.
Cited by
-
Occurrence of Diarrhea and Feeding Practices among Children below Two Years of Age in Southwestern Saudi Arabia.Int J Environ Res Public Health. 2020 Jan 22;17(3):722. doi: 10.3390/ijerph17030722. Int J Environ Res Public Health. 2020. PMID: 31979127 Free PMC article.
-
The economic burden of rotavirus hospitalization among children < 5 years of age in selected hospitals in Bangladesh.Vaccine. 2021 Nov 26;39(48):7082-7090. doi: 10.1016/j.vaccine.2021.10.003. Epub 2021 Oct 30. Vaccine. 2021. PMID: 34756769 Free PMC article.
-
Comparative Analysis of G1P[8] Rotaviruses Identified Prior to Vaccine Implementation in Pakistan With Rotarix™ and RotaTeq™ Vaccine Strains.Front Immunol. 2020 Oct 2;11:562282. doi: 10.3389/fimmu.2020.562282. eCollection 2020. Front Immunol. 2020. PMID: 33133073 Free PMC article.
-
Treatment-seeking and recovery among young undernourished children post-hospital discharge in Bangladesh: A qualitative study.PLoS One. 2022 Sep 23;17(9):e0274996. doi: 10.1371/journal.pone.0274996. eCollection 2022. PLoS One. 2022. PMID: 36149880 Free PMC article.
References
-
- Madhi SA, Cunliffe NA, Steele D, et al. . Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362:289–98. - PubMed
-
- Armah GE, Sow SO, Breiman RF, et al. . Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376:606–14. - PubMed
-
- International Vaccine Access Center Johns Hopkins Bloomberg School of Public Health. VIEW-hub global vaccine introduction and implementation report. Available at: www.jhsph.edu/ivac/view-hub. Accessed 23 March 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

