Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn's Disease Studies
- PMID: 30753371
- PMCID: PMC6535500
- DOI: 10.1093/ecco-jcc/jjy223
Efficacy and Safety of Adalimumab by Disease Duration: Analysis of Pooled Data From Crohn's Disease Studies
Abstract
Background and aims: Analyses of Crohn's Disease [CD] studies of anti-TNF agents, including adalimumab, have reported higher remission rates among patients with shorter disease duration. To further explore the relationship between disease duration and clinical efficacy, we analysed a larger patient cohort.
Methods: Data were pooled from 10 clinical trials in patients with moderately to severely active CD who received treatment with either adalimumab or placebo. Analyses of efficacy using Crohn's Disease Activity Index [CDAI] endpoints [remission, clinical response [CR]-70, CR-100, patient-reported outcome [PRO] remission] or Harvey-Bradshaw Index [HBI] endpoints [remission/response] were conducted for induction and maintenance treatment periods. Logistic regression was used for comparisons between adalimumab and placebo treatment. Cochran-Armitage trend tests were used for comparisons between disease-duration subgroups [<1 year, ≥1-<2 years, 2-≤5 years, and >5 years].
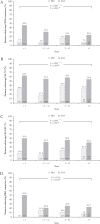
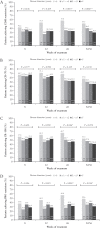
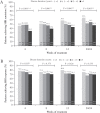
Results: During induction, the proportion of patients achieving CDAI remission was higher in adalimumab- versus placebo-treated patients [p <0.001] and was highest [adalimumab: 45.8%] in the <1 year subgroup compared with longer disease-duration subgroups [≥1-<2 years: 31.0%; 2-≤5 years: 23.1%; >5 years: 23.6%, Cochran-Armitage p = 0.026]. In the majority of maintenance treatment analyses, patients with <1 year disease duration had the highest efficacy responses, with statistically significant differences in remission rates across disease-duration subgroups.
Conclusions: This analysis demonstrates that earlier initiation of adalimumab treatment shortly after diagnosis in patients with moderately to severely active CD leads to improved long-term clinical outcomes.
Keywords: Adalimumab; Crohn’s disease; disease duration.
© European Crohn’s and Colitis Organisation (ECCO) 2019.
Figures
References
-
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. - PubMed
-
- Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology 2017;152:351–61.e5. - PubMed
-
- Gomollón F, Dignass A, Annese V, et al. ; ECCO. Third European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016. Part 1: diagnosis and medical management. J Crohns Colitis 2017;11:3–25. - PubMed
-
- Hazlewood GS, Rezaie A, Borman M, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015;148:344–54.e5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

