Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers
- PMID: 30753379
- PMCID: PMC6439328
- DOI: 10.1093/brain/awz019
Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers
Abstract
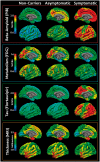
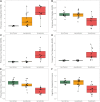
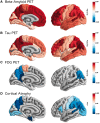
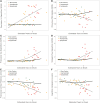
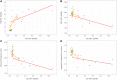
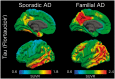
Tauopathy is a hallmark pathology of Alzheimer's disease with a strong relationship with cognitive impairment. As such, understanding tau may be a key to clinical interventions. In vivo tauopathy has been measured using cerebrospinal fluid assays, but these do not provide information about where pathology is in the brain. The introduction of PET ligands that bind to paired helical filaments provides the ability to measure the amount and distribution of tau pathology. The heritability of the age of dementia onset tied to the specific mutations found in autosomal dominant Alzheimer's disease families provides an elegant model to study the spread of tau across the course of the disease as well as the cross-modal relationship between tau and other biomarkers. To better understand the pathobiology of Alzheimer's disease we measured levels of tau PET binding in individuals with dominantly inherited Alzheimer's disease using data from the Dominantly Inherited Alzheimer Network (DIAN). We examined cross-sectional measures of amyloid-β, tau, glucose metabolism, and grey matter degeneration in 15 cognitively normal mutation non-carriers, 20 asymptomatic carriers, and 15 symptomatic mutation carriers. Linear models examined the association of pathology with group, estimated years to symptom onset, as well as cross-modal relationships. For comparison, tau PET was acquired on 17 older adults with sporadic, late onset Alzheimer disease. Tau PET binding was starkly elevated in symptomatic DIAN individuals throughout the cortex. The brain areas demonstrating elevated tau PET binding overlapped with those seen in sporadic Alzheimer's disease, but with a greater cortical involvement and greater levels of binding despite similar cognitive impairment. Tau PET binding was elevated in the temporal lobe, but the most prominent loci of pathology were in the precuneus and lateral parietal regions. Symptomatic mutation carriers also demonstrated elevated tau PET binding in the basal ganglia, consistent with prior work with amyloid-β. The degree of tau tracer binding in symptomatic individuals was correlated to other biomarkers, particularly markers of neurodegeneration. In addition to the differences seen with tau, amyloid-β was increased in both asymptomatic and symptomatic groups relative to non-carriers. Glucose metabolism showed decline primarily in the symptomatic group. MRI indicated structural degeneration in both asymptomatic and symptomatic cohorts. We demonstrate that tau PET binding is elevated in symptomatic individuals with dominantly inherited Alzheimer's disease. Tau PET uptake was tied to the onset of cognitive dysfunction, and there was a higher amount, and different regional pattern of binding compared to late onset, non-familial Alzheimer's disease.
Keywords: Alzheimer’s; FDG; MRI; amyloid; flortaucipir.
© The Author(s) (2019). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Characterizing brain tau and cognitive decline along the amyloid timeline in Alzheimer's disease.Brain. 2024 Jun 3;147(6):2144-2157. doi: 10.1093/brain/awae116. Brain. 2024. PMID: 38667631 Free PMC article.
-
Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages.Brain. 2021 Dec 16;144(11):3517-3528. doi: 10.1093/brain/awab248. Brain. 2021. PMID: 34515754 Free PMC article.
-
Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease.Brain. 2016 May;139(Pt 5):1551-67. doi: 10.1093/brain/aww027. Epub 2016 Mar 8. Brain. 2016. PMID: 26962052 Free PMC article.
-
In vivo tau PET imaging in dementia: Pathophysiology, radiotracer quantification, and a systematic review of clinical findings.Ageing Res Rev. 2017 Jul;36:50-63. doi: 10.1016/j.arr.2017.03.002. Epub 2017 Mar 15. Ageing Res Rev. 2017. PMID: 28315409
-
Biological and Cognitive Markers of Presenilin1 E280A Autosomal Dominant Alzheimer's Disease: A Comprehensive Review of the Colombian Kindred.J Prev Alzheimers Dis. 2019;6(2):112-120. doi: 10.14283/jpad.2019.6. J Prev Alzheimers Dis. 2019. PMID: 30756118 Free PMC article. Review.
Cited by
-
Tau as a biomarker of cognitive impairment and neuropsychiatric symptom in Alzheimer's disease.Hum Brain Mapp. 2023 Feb 1;44(2):327-340. doi: 10.1002/hbm.26043. Epub 2022 Aug 14. Hum Brain Mapp. 2023. PMID: 36647262 Free PMC article.
-
Role of tau deposition in early cognitive decline in Down syndrome.Alzheimers Dement (Amst). 2022 Apr 1;14(1):e12256. doi: 10.1002/dad2.12256. eCollection 2022. Alzheimers Dement (Amst). 2022. PMID: 35386473 Free PMC article.
-
Serum neurofilament light chain levels are associated with white matter integrity in autosomal dominant Alzheimer's disease.Neurobiol Dis. 2020 Aug;142:104960. doi: 10.1016/j.nbd.2020.104960. Epub 2020 Jun 6. Neurobiol Dis. 2020. PMID: 32522711 Free PMC article.
-
Cortical hypometabolism reflects local atrophy and tau pathology in symptomatic Alzheimer's disease.Brain. 2022 Apr 18;145(2):713-728. doi: 10.1093/brain/awab294. Brain. 2022. PMID: 34373896 Free PMC article.
-
Comparison of tau spread in people with Down syndrome versus autosomal-dominant Alzheimer's disease: a cross-sectional study.Lancet Neurol. 2024 May;23(5):500-510. doi: 10.1016/S1474-4422(24)00084-X. Lancet Neurol. 2024. PMID: 38631766 Free PMC article.
References
-
- Arriagada PV, Growdon JH, Hedley-whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 1992; 42: 631. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995; 57: 289–300.
Publication types
MeSH terms
Substances
Grants and funding
- MR/L023784/1/MRC_/Medical Research Council/United Kingdom
- U01 AG032438/AG/NIA NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- TL1 TR002344/TR/NCATS NIH HHS/United States
- K01 AG053474/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- MR/009076/1/MRC_/Medical Research Council/United Kingdom
- R01 AG046179/AG/NIA NIH HHS/United States
- S10 RR022984/RR/NCRR NIH HHS/United States
- R01 EB009352/EB/NIBIB NIH HHS/United States
- R01 AG053267/AG/NIA NIH HHS/United States
- P30 NS098577/NS/NINDS NIH HHS/United States
- R01 AG052550/AG/NIA NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States
- U01 AG042791/AG/NIA NIH HHS/United States
- S10 OD018091/OD/NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- UF1 AG032438/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

