Accumulation of EBI3 induced by virulent Mycobacterium tuberculosis inhibits apoptosis in murine macrophages
- PMID: 30753412
- PMCID: PMC6414311
- DOI: 10.1093/femspd/ftz007
Accumulation of EBI3 induced by virulent Mycobacterium tuberculosis inhibits apoptosis in murine macrophages
Abstract
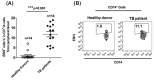
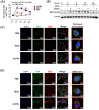
Macrophages are the primary host target cells of Mycobacterium tuberculosis (M. tb). As a subunit of immunoregulatory cytokines IL-27 and IL-35, Epstein-Barr virus-induced gene 3 (EBI3) has typically been explored as the secreted form and assessed in terms of its effects triggered by extracellular EBI3. However, little is known about intracellular EBI3 function. In the current study, we report that EBI3 production by macrophages is elevated in TB patients. We further demonstrate that increased EBI3 accumulates in virulent M. tb-treated murine macrophages. Eukaryotic translation elongation factor 1-alpha 1 (eEF1A1) binds to intracellular EBI3 to reduce Lys48 (K48)-linked ubiquitination of EBI3, leading to EBI3 accumulation. Moreover, the intracellular EBI3 inhibits caspase-3-mediated apoptosis in M. tb-treated macrophages. Herein, we propose a novel mechanism for accumulating intracellular EBI3 and its regulation of macrophage apoptosis in response to virulent M. tb.
Keywords: M. tb; EBI3; apoptosis; eEF1A1; macrophage; ubiquitination.
© FEMS 2019.
Figures




References
-
- Bohme J, Rossnagel C, Jacobs T et al. .. Epstein-Barr virus-induced gene 3 suppresses T helper type 1, type 17 and type 2 immune responses after Trypanosoma cruzi infection and inhibits parasite replication by interfering with alternative macrophage activation. Immunology. 2016;147: 338–48. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

