Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate
- PMID: 30753464
- PMCID: PMC6389866
- DOI: 10.1093/humrep/dey373
Complete spermatogenesis in intratesticular testis tissue xenotransplants from immature non-human primate
Abstract
Study question: Can full spermatogenesis be achieved after xenotransplantation of prepubertal primate testis tissue to the mouse, in testis or subcutaneously?
Summary answer: Intratesticular xenotransplantation supported the differentiation of immature germ cells from marmoset (Callithrix jacchus) into spermatids and spermatozoa at 4 and 9 months post-transplantation, while in subcutaneous transplants, spermatogenic arrest was observed at 4 months and none of the transplants survived at 9 months.
What is known already: Auto-transplantation of cryopreserved immature testis tissue (ITT) could be a potential fertility restoration strategy for patients with complete loss of germ cells due to chemo- and/or radiotherapy at a young age. Before ITT transplantation can be used for clinical application, it is a prerequisite to demonstrate the feasibility of the technique and identify the conditions required for establishing spermatogenesis in primate ITT transplants. Although xenotransplantation of ITT from several species has resulted in complete spermatogenesis, in human and marmoset, ITT has not been successful.
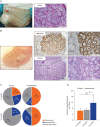
Study design, size, duration: In this study, we used marmoset as a pre-clinical animal model. ITT was obtained from two 6-month-old co-twin marmosets. A total of 147 testis tissue pieces (~0.8-1.0 mm3 each) were transplanted into the testicular parenchyma (intratesticular; n = 40) or under the dorsal skin (ectopic; n = 107) of 4-week-old immunodeficient Swiss Nu/Nu mice (n = 20). Each mouse received one single marmoset testis tissue piece in each testis and 4-6 pieces subcutaneously. Xenotransplants were retrieved at 4 and 9 months post-transplantation and evaluations were performed with regards to transplant survival, spermatogonial quantity and germ cell differentiation.
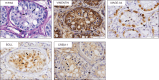
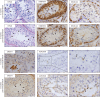
Participants/materials, setting, methods: Transplant survival was histologically evaluated by haematoxylin-periodic acid Schiff (H/PAS) staining. Spermatogonia were identified by MAGE-A4 via immunohistochemistry. Germ cell differentiation was assessed by morphological identification of different germ cell types on H/PAS stained sections. Meiotically active germ cells were identified by BOLL expression. CREM immunohistochemistry was performed to confirm the presence of post-meiotic germ cells and ACROSIN was used to determine the presence of round, elongating and elongated spermatids.
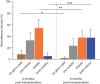
Main results and the role of chance: Four months post-transplantation, 50% of the intratesticular transplants and 21% of the ectopic transplants were recovered (P = 0.019). The number of spermatogonia per tubule did not show any variation. In 33% of the recovered intratesticular transplants, complete spermatogenesis was established. Overall, 78% of the intratesticular transplants showed post-meiotic differentiation (round spermatids, elongating/elongated spermatids and spermatozoa). However, during the same period, spermatocytes (early meiotic germ cells) were the most advanced germ cell type present in the ectopic transplants. Nine months post-transplantation, 50% of the intratesticular transplants survived, whilst none of the ectopic transplants was recovered (P < 0.0001). Transplants contained more spermatogonia per tubule (P = 0.018) than at 4 months. Complete spermatogenesis was observed in all recovered transplants (100%), indicating a progressive spermatogenic development in intratesticular transplants between the two time-points. Nine months post-transplantation, transplants contained more seminiferous tubules with post-meiotic germ cells (37 vs. 5%; P < 0.001) and fewer tubules without germ cells (2 vs. 8%; P = 0.014) compared to 4 months post-transplantation.
Large scale data: N/A.
Limitations, reasons for caution: Although xenotransplantation of marmoset ITT was successful, it does not fully reflect all aspects of a future clinical setting. Furthermore, due to ethical restrictions, we were not able to prove the functionality of the spermatozoa produced in the marmoset transplants.
Wider implications of the findings: In this pre-clinical study, we demonstrated that testicular parenchyma provides the required microenvironment for germ cell differentiation and long-term survival of immature marmoset testis tissue, likely due to the favourable temperature regulation, growth factors and hormonal support. These results encourage the design of new experiments on human ITT xenotransplantation and show that intratesticular transplantation is likely to be superior to ectopic transplantation for fertility restoration following gonadotoxic treatment in childhood.
Study funding/competing interest(s): This project was funded by the ITN Marie Curie Programme 'Growsperm' (EU-FP7-PEOPLE-2013-ITN 603568) and the scientific Fund Willy Gepts from the UZ Brussel (ADSI677). D.V.S. is a post-doctoral fellow of the Fonds Wetenschappelijk Onderzoek (FWO; 12M2815N). No conflict of interest is declared.
Keywords: fertility preservation; immature testis tissue; intratesticular transplantation; primates; spermatogenesis.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures

Similar articles
-
When does germ cell loss and fibrosis occur in patients with Klinefelter syndrome?Hum Reprod. 2018 Jun 1;33(6):1009-1022. doi: 10.1093/humrep/dey094. Hum Reprod. 2018. PMID: 29684126
-
Assessment of fresh and cryopreserved testicular tissues from (pre)pubertal boys during organ culture as a strategy for in vitro spermatogenesis.Hum Reprod. 2019 Dec 1;34(12):2443-2455. doi: 10.1093/humrep/dez180. Hum Reprod. 2019. PMID: 31858131 Free PMC article.
-
Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue.Mol Hum Reprod. 2017 Nov 1;23(11):738-754. doi: 10.1093/molehr/gax054. Mol Hum Reprod. 2017. PMID: 29040674
-
Fertility preservation for prepubertal boys: lessons learned from the past and update on remaining challenges towards clinical translation.Hum Reprod Update. 2021 Apr 21;27(3):433-459. doi: 10.1093/humupd/dmaa050. Hum Reprod Update. 2021. PMID: 33326572 Review.
-
Review on testicular development, structure, function, and regulation in common marmoset.Birth Defects Res B Dev Reprod Toxicol. 2005 Oct;74(5):450-69. doi: 10.1002/bdrb.20057. Birth Defects Res B Dev Reprod Toxicol. 2005. PMID: 16193499 Review.
Cited by
-
The Role of Promyelocytic Leukemia Zinc Finger (PLZF) and Glial-Derived Neurotrophic Factor Family Receptor Alpha 1 (GFRα1) in the Cryopreservation of Spermatogonia Stem Cells.Int J Mol Sci. 2023 Jan 18;24(3):1945. doi: 10.3390/ijms24031945. Int J Mol Sci. 2023. PMID: 36768269 Free PMC article. Review.
-
Biomaterials for Testicular Bioengineering: How far have we come and where do we have to go?Front Endocrinol (Lausanne). 2023 Mar 16;14:1085872. doi: 10.3389/fendo.2023.1085872. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37008920 Free PMC article. Review.
-
In Vitro Generation of Haploid Germ Cells from Human XY and XXY Immature Testes in a 3D Organoid System.Bioengineering (Basel). 2024 Jul 3;11(7):677. doi: 10.3390/bioengineering11070677. Bioengineering (Basel). 2024. PMID: 39061759 Free PMC article.
-
Immunological microenvironment in the testis.Reprod Med Biol. 2019 Aug 29;19(1):24-31. doi: 10.1002/rmb2.12293. eCollection 2020 Jan. Reprod Med Biol. 2019. PMID: 31956282 Free PMC article. Review.
-
Pediatric oncofertility: an update.Transl Androl Urol. 2020 Oct;9(5):2416-2421. doi: 10.21037/tau-20-991. Transl Androl Urol. 2020. PMID: 33209715 Free PMC article. Review.
References
-
- Abrishami M, Abbasi S, Honaramooz A. The effect of donor age on progression of spermatogenesis in canine testicular tissue after xenografting into immunodeficient mice. Theriogenology 2010;73:512–522. - PubMed
-
- Behr R, Weinbauer GF. cAMP response element modulator (CREM): an essential factor for spermatogenesis in primates? Int J Androl 2001;24:126–135. - PubMed
-
- de Michele F, Poels J, Weerens L, Petit C, Evrard Z, Ambroise J, Gruson D, Wyns C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of Sertoli and Leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum Reprod 2017;32:32–45. - PubMed
-
- Geens M, de Block G, Goossens E, Frederickx V, van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum Reprod 2006;21:390–396. - PubMed
-
- Giudice MG, de Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: how far are we from clinical practice? Stem Cell Res 2017;21:171–177. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

