Immunotherapy for skin cancer
- PMID: 30753483
- PMCID: PMC6626298
- DOI: 10.1093/intimm/dxz012
Immunotherapy for skin cancer
Abstract
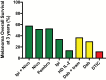
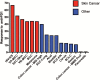
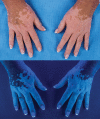
Among all tumor types, skin cancers are profoundly sensitive to immunotherapy. Indeed, the recently reported response rates for anti-PD-1 (anti-programmed-death 1) therapy for cutaneous malignant melanomas (MM), Merkel cell carcinomas, basal cell carcinomas, cutaneous squamous cell carcinomas and Kaposi sarcomas are all above 40%. This unique immunogenicity renders skin cancers as a paradigm for tumor-immune interactions and is driven by high mutational burdens, over-expressed tumor antigens and/or viral antigens. However, despite the clear demonstration of immunologic cure of skin cancer in some patients, most tumors develop either early (primary) or late (adaptive) resistance to immunotherapy. Resistance mechanisms are complex, and include contributions of tumor cell-intrinsic, T cell and microenvironment factors that have been recently further elucidated with the advent of single-cell technologies. This review will focus on the exciting progress with immunotherapy for skin cancers to date, and also our current understanding of the mechanisms of resistance to immunotherapy.
Keywords: Merkel cell; PD-1; cutaneous malignancy; immune checkpoint; melanoma.
© The Japanese Society for Immunology. 2019. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Siegel R. L., Miller K. D. and Jemal A. 2018. Cancer statistics, 2018. CA Cancer J. Clin. 68:7. - PubMed
-
- Rogers H. W., Weinstock M. A., Feldman S. R. and Coldiron B. M. 2015. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 151:1081. - PubMed
-
- Robert C., Thomas L., Bondarenko I. et al. 2011. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364:2517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

