Glutamatergic Neurokinin 3 Receptor Neurons in the Median Preoptic Nucleus Modulate Heat-Defense Pathways in Female Mice
- PMID: 30753503
- PMCID: PMC6424091
- DOI: 10.1210/en.2018-00934
Glutamatergic Neurokinin 3 Receptor Neurons in the Median Preoptic Nucleus Modulate Heat-Defense Pathways in Female Mice
Abstract
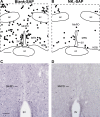
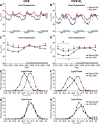
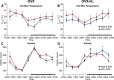
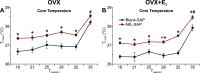
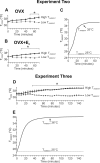
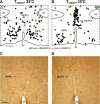
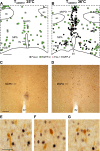
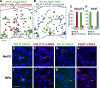
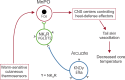
We have proposed that arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin (KNDy neurons) contribute to hot flushes via projections to neurokinin 3 receptor (NK3R)-expressing neurons in the median preoptic nucleus (MnPO). To characterize the thermoregulatory role of MnPO NK3R neurons in female mice, we ablated these neurons using injections of saporin toxin conjugated to a selective NK3R agonist. Loss of MnPO NK3R neurons increased the core temperature (TCORE) during the light phase, with the frequency distributions indicating a regulated shift in the balance point. The increase in TCORE in the ablated mice occurred despite changes in the ambient temperature and regardless of estrogen status. We next determined whether an acute increase in ambient temperature or higher TCORE would induce Fos in preoptic enhanced green fluorescent protein (EGFP)-immunoreactive neurons in Tacr3-EGFP mice. Fos activation was increased in the MnPO but no induction of Fos was found in NK3R (EGFP-immunoreactive) neurons. Thus, MnPO NK3R neurons are not activated by warm thermosensors in the skin or viscera and are not warm-sensitive neurons. Finally, RNAscope was used to determine whether Tacr3 (NK3R) mRNA was coexpressed with vesicular glutamate transporter 2 or vesicular γ-aminobutyric acid (GABA) transporter mRNA, markers of glutamatergic and GABAergic neurotransmission, respectively. In the MnPO, 94% of NK3R neurons were glutamatergic, but in the adjacent medial preoptic area, 97% of NK3R neurons were GABAergic. Thus, NK3R neurons in the MnPO are glutamatergic and play a role in reducing TCORE but are not activated by warm thermal stimuli (internal or external). These findings suggest that KNDy neurons modulate thermosensory pathways for heat defense indirectly via a subpopulation of glutamatergic MnPO neurons that express NK3R.
Copyright © 2019 Endocrine Society.
Figures
Similar articles
-
Neurokinin 3 Receptor-Expressing Neurons in the Median Preoptic Nucleus Modulate Heat-Dissipation Effectors in the Female Rat.Endocrinology. 2015 Jul;156(7):2552-62. doi: 10.1210/en.2014-1974. Epub 2015 Mar 31. Endocrinology. 2015. PMID: 25825817 Free PMC article.
-
Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat.Endocrinology. 2011 Dec;152(12):4894-905. doi: 10.1210/en.2011-1492. Epub 2011 Oct 25. Endocrinology. 2011. PMID: 22028440 Free PMC article.
-
Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes.Front Neuroendocrinol. 2013 Aug;34(3):211-27. doi: 10.1016/j.yfrne.2013.07.003. Epub 2013 Jul 17. Front Neuroendocrinol. 2013. PMID: 23872331 Free PMC article. Review.
-
Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice.Endocrinology. 2013 Aug;154(8):2761-71. doi: 10.1210/en.2013-1268. Epub 2013 Jun 6. Endocrinology. 2013. PMID: 23744642 Free PMC article.
-
The neuroendocrinology of the preoptic area in menopause: Symptoms and therapeutic strategies.Handb Clin Neurol. 2021;179:455-460. doi: 10.1016/B978-0-12-819975-6.00029-7. Handb Clin Neurol. 2021. PMID: 34225982 Review.
Cited by
-
Hypothalamic Kisspeptin Neurons and the Control of Homeostasis.Endocrinology. 2022 Feb 1;163(2):bqab253. doi: 10.1210/endocr/bqab253. Endocrinology. 2022. PMID: 34953135 Free PMC article. Review.
-
A phase 2b, randomized, placebo-controlled, double-blind, dose-ranging study of the neurokinin 3 receptor antagonist fezolinetant for vasomotor symptoms associated with menopause.Menopause. 2020 Apr;27(4):382-392. doi: 10.1097/GME.0000000000001510. Menopause. 2020. PMID: 32102086 Free PMC article. Clinical Trial.
-
Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice.Nat Commun. 2020 Dec 11;11(1):6378. doi: 10.1038/s41467-020-20050-1. Nat Commun. 2020. PMID: 33311503 Free PMC article.
-
Neural substrates underlying rhythmic coupling of female reproductive and thermoregulatory circuits.Front Physiol. 2023 Sep 11;14:1254287. doi: 10.3389/fphys.2023.1254287. eCollection 2023. Front Physiol. 2023. PMID: 37753455 Free PMC article. Review.
-
Efficacy and Safety of Fezolinetant in Moderate to Severe Vasomotor Symptoms Associated With Menopause: A Phase 3 RCT.J Clin Endocrinol Metab. 2023 Jul 14;108(8):1981-1997. doi: 10.1210/clinem/dgad058. J Clin Endocrinol Metab. 2023. PMID: 36734148 Free PMC article. Clinical Trial.
References
-
- Santoro N. Symptoms of menopause: hot flushes. Clin Obstet Gynecol. 2008;51(3):539–548. - PubMed
-
- Kronenberg F. Menopausal hot flashes: a review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140(7):1380S–1385S. - PubMed
-
- Freedman RR. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–464. - PubMed
-
- Rance NE, Young WS III. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. - PubMed
-
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

