Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects
- PMID: 30753544
- PMCID: PMC6548897
- DOI: 10.1093/infdis/jiz064
Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects
Abstract
Background: Metformin, the most widely administered diabetes drug, has been proposed as a candidate adjunctive host-directed therapy for tuberculosis, but little is known about its effects on human host responses to Mycobacterium tuberculosis.
Methods: We investigated in vitro and in vivo effects of metformin in humans.
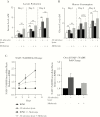
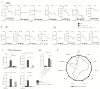
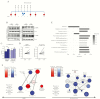
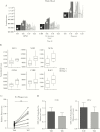
Results: Metformin added to peripheral blood mononuclear cells from healthy volunteers enhanced in vitro cellular metabolism while inhibiting the mammalian target of rapamycin targets p70S6K and 4EBP1, with decreased cytokine production and cellular proliferation and increased phagocytosis activity. Metformin administered to healthy human volunteers led to significant downregulation of genes involved in oxidative phosphorylation, mammalian target of rapamycin signaling, and type I interferon response pathways, particularly following stimulation with M. tuberculosis, and upregulation of genes involved in phagocytosis and reactive oxygen species production was increased. These in vivo effects were accompanied by a metformin-induced shift in myeloid cells from classical to nonclassical monocytes. At a functional level, metformin lowered ex vivo production of tumor necrosis factor α, interferon γ, and interleukin 1β but increased phagocytosis activity and reactive oxygen species production.
Conclusion: Metformin has a range of potentially beneficial effects on cellular metabolism, immune function, and gene transcription involved in innate host responses to M. tuberculosis.
Keywords: Metformin; antimycobacterial mechanisms; gene transcription; host-directed therapy; tuberculosis.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


Similar articles
-
Glibenclamide alters interleukin-8 and interleukin-1β of primary human monocytes from diabetes patients against Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2020 Jul;123:101939. doi: 10.1016/j.tube.2020.101939. Epub 2020 May 15. Tuberculosis (Edinb). 2020. PMID: 32452426
-
TREM2 Promotes Immune Evasion by Mycobacterium tuberculosis in Human Macrophages.mBio. 2022 Aug 30;13(4):e0145622. doi: 10.1128/mbio.01456-22. Epub 2022 Aug 4. mBio. 2022. PMID: 35924849 Free PMC article.
-
Metformin has immunomodulatory effects which support its potential use as adjunctive therapy in tuberculosis.Indian J Tuberc. 2024 Jan;71(1):89-95. doi: 10.1016/j.ijtb.2023.05.011. Epub 2023 May 16. Indian J Tuberc. 2024. PMID: 38296396 Review.
-
Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis.Infect Immun. 1996 Jul;64(7):2523-31. doi: 10.1128/iai.64.7.2523-2531.1996. Infect Immun. 1996. PMID: 8698475 Free PMC article.
-
Metformin: Candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients.Tuberculosis (Edinb). 2016 Dec;101S:S69-S72. doi: 10.1016/j.tube.2016.09.008. Epub 2016 Sep 28. Tuberculosis (Edinb). 2016. PMID: 27720378 Review.
Cited by
-
Immunopathogenic overlap between COVID-19 and tuberculosis identified from transcriptomic meta-analysis and human macrophage infection.iScience. 2022 May 25;25(6):104464. doi: 10.1016/j.isci.2022.104464. eCollection 2022 Jun 17. iScience. 2022. PMID: 35634577 Free PMC article.
-
Mycobacterium tuberculosis: Pathogenesis and therapeutic targets.MedComm (2020). 2023 Sep 4;4(5):e353. doi: 10.1002/mco2.353. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37674971 Free PMC article. Review.
-
Progress and applications of mass cytometry in sketching immune landscapes.Clin Transl Med. 2020 Oct;10(6):e206. doi: 10.1002/ctm2.206. Clin Transl Med. 2020. PMID: 33135337 Free PMC article. Review.
-
Impact of metformin on the risk and treatment outcomes of tuberculosis in diabetics: a systematic review.BMC Infect Dis. 2019 Oct 17;19(1):859. doi: 10.1186/s12879-019-4548-4. BMC Infect Dis. 2019. PMID: 31623569 Free PMC article.
-
Potentials of Host-Directed Therapies in Tuberculosis Management.J Clin Med. 2019 Aug 3;8(8):1166. doi: 10.3390/jcm8081166. J Clin Med. 2019. PMID: 31382631 Free PMC article. Review.
References
-
- Singhal A, Jie L, Kumar P, et al. . Metformin as adjunct antituberculosis therapy. Sci Transl Med 2014; 6:263ra159. - PubMed
-
- Leow MK, Dalan R, Chee CB, et al. . Latent tuberculosis in patients with diabetes mellitus: prevalence, progression and public health implications. Exp Clin Endocrinol Diabetes 2014; 122:528–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

