Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients
- PMID: 30753611
- PMCID: PMC6620642
- DOI: 10.1093/neuonc/noz040
Phase I/II trial testing safety and immunogenicity of the multipeptide IMA950/poly-ICLC vaccine in newly diagnosed adult malignant astrocytoma patients
Abstract
Background: Peptide vaccines offer the opportunity to elicit glioma-specific T cells with tumor killing ability. Using antigens eluted from the surface of glioblastoma samples, we designed a phase I/II study to test safety and immunogenicity of the IMA950 multipeptide vaccine adjuvanted with poly-ICLC (polyinosinic-polycytidylic acid stabilized with polylysine and carboxymethylcellulose) in human leukocyte antigen A2+ glioma patients.
Methods: Adult patients with newly diagnosed glioblastoma (n = 16) and grade III astrocytoma (n = 3) were treated with radiochemotherapy followed by IMA950/poly-ICLC vaccination. The first 6 patients received IMA950 (9 major histocompatibility complex [MHC] class I and 2 MHC class II peptides) intradermally and poly-ICLC intramuscularly (i.m.). After protocol amendment, IMA950 and poly-ICLC were mixed and injected subcutaneously (n = 7) or i.m. (n = 6). Primary endpoints were safety and immunogenicity. Secondary endpoints were overall survival, progression-free survival at 6 and 9 months, and vaccine-specific peripheral cluster of differentiation (CD)4 and CD8 T-cell responses.
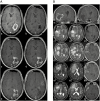
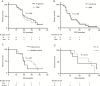
Results: The IMA950/poly-ICLC vaccine was safe and well tolerated. Four patients presented cerebral edema with rapid recovery. For the first 6 patients, vaccine-induced CD8 T-cell responses were restricted to a single peptide and CD4 responses were absent. After optimization of vaccine formulation, we observed multipeptide CD8 and sustained T helper 1 CD4 T-cell responses. For the entire cohort, CD8 T-cell responses to a single or multiple peptides were observed in 63.2% and 36.8% of patients, respectively. Median overall survival was 19 months for glioblastoma patients.
Conclusion: We provide, in a clinical trial, using cell surface-presented antigens, insights into optimization of vaccines generating effector T cells for glioma patients.
Trial registration: Clinicaltrials.gov NCT01920191.
Keywords: IMA950; glioma; immune response; peptide vaccine; poly-ICLC.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma.J Clin Oncol. 2011 Jan 20;29(3):330-6. doi: 10.1200/JCO.2010.30.7744. Epub 2010 Dec 13. J Clin Oncol. 2011. PMID: 21149657 Free PMC article. Clinical Trial.
-
Induction of robust type-I CD8+ T-cell responses in WHO grade 2 low-grade glioma patients receiving peptide-based vaccines in combination with poly-ICLC.Clin Cancer Res. 2015 Jan 15;21(2):286-94. doi: 10.1158/1078-0432.CCR-14-1790. Epub 2014 Nov 25. Clin Cancer Res. 2015. PMID: 25424847 Free PMC article. Clinical Trial.
-
Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients.Clin Cancer Res. 2012 Dec 1;18(23):6497-508. doi: 10.1158/1078-0432.CCR-12-2189. Epub 2012 Oct 2. Clin Cancer Res. 2012. PMID: 23032745 Clinical Trial.
-
Brain tumor immunotherapy with type-1 polarizing strategies.Ann N Y Acad Sci. 2009 Sep;1174:18-23. doi: 10.1111/j.1749-6632.2009.04932.x. Ann N Y Acad Sci. 2009. PMID: 19769732 Review.
-
Poly(I:C) as cancer vaccine adjuvant: knocking on the door of medical breakthroughs.Pharmacol Ther. 2015 Feb;146:120-31. doi: 10.1016/j.pharmthera.2014.09.010. Epub 2014 Oct 2. Pharmacol Ther. 2015. PMID: 25281915 Review.
Cited by
-
Introduction to immunotherapy for brain tumor patients: challenges and future perspectives.Neurooncol Pract. 2020 Mar 9;7(5):465-476. doi: 10.1093/nop/npaa007. eCollection 2020 Oct. Neurooncol Pract. 2020. PMID: 33014387 Free PMC article. Review.
-
Citrullinated Epitopes Identified on Tumour MHC Class II by Peptide Elution Stimulate Both Regulatory and Th1 Responses and Require Careful Selection for Optimal Anti-Tumour Responses.Front Immunol. 2021 Nov 9;12:764462. doi: 10.3389/fimmu.2021.764462. eCollection 2021. Front Immunol. 2021. PMID: 34858415 Free PMC article.
-
Personalised therapeutic approaches to glioblastoma: A systematic review.Front Med (Lausanne). 2023 Apr 14;10:1166104. doi: 10.3389/fmed.2023.1166104. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37122327 Free PMC article.
-
New insights for the development of efficient DNA vaccines.Microb Biotechnol. 2024 Nov;17(11):e70053. doi: 10.1111/1751-7915.70053. Microb Biotechnol. 2024. PMID: 39545748 Free PMC article. Review.
-
Immunotherapy for glioblastoma: current state, challenges, and future perspectives.Cell Mol Immunol. 2024 Dec;21(12):1354-1375. doi: 10.1038/s41423-024-01226-x. Epub 2024 Oct 15. Cell Mol Immunol. 2024. PMID: 39406966 Free PMC article. Review.
References
-
- Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- Garon EB, Rizvi NA, Hui R, et al. ; KEYNOTE-001 Investigators Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. - PubMed
-
- Robert C, Schachter J, Long GV, et al. ; KEYNOTE-006 investigators Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

