Trends in BRCA Test Utilization in an Integrated Health System, 2005-2015
- PMID: 30753636
- PMCID: PMC6695306
- DOI: 10.1093/jnci/djz008
Trends in BRCA Test Utilization in an Integrated Health System, 2005-2015
Abstract
Background: Genetic testing to determine BRCA status has been available for over two decades, but there are few population-based studies of test diffusion. We report 10-year trends in BRCAtesting in an integrated health-care system with long-standing access to genetic services.
Methods: A cohort of women aged 18 years and older was created to ascertain BRCA testing (n = 295 087). Annual testing rates between 2005 and 2015 were calculated in all women with and without incident (ie, newly diagnosed) breast and ovarian cancers and in clinically eligible subgroups by family cancer history, personal cancer history, and age at diagnosis. Secular trends were assessed using Poisson regression. Women tested early (2005-2008), midway (2009-2012), and late (2013-2015) in the study period were compared in cross-sectional analyses.
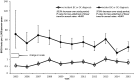
Results: Between 2005 and 2015, annual testing rates increased from 0.6/1000 person-years (pys) (95% confidence interval [CI] = 0.4 to 0.7/1000 pys) to 0.8/1000 pys (95% CI = 0.6 to 1.0/1000 pys) in women without incident breast or ovarian cancers. Rates decreased from 71.5/1000 pys (95% CI = 42.4 to 120.8/1000 pys) to 44.4/1000 pys (95% CI = 35.5 to 55.6/1000 pys) in women with incident diagnoses, despite improvements in provision of timely BRCA testing during this time frame. We found no evidence of secular trends in clinically eligible subgroups including women with family history indicating increased hereditary cancer risk, but no personal cancer history. At the end of the study period, 97.0% (95% CI = 96.6% to 97.3%) of these women remained untested.
Conclusion: Many eligible women did not receive BRCA testing despite having insurance coverage and access to specialty genetic services, underscoring challenges to primary and secondary hereditary cancer prevention.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Comment in
-
Persistent Underutilization of BRCA1/2 Testing Suggest the Need for New Approaches to Genetic Testing Delivery.J Natl Cancer Inst. 2019 Aug 1;111(8):751-753. doi: 10.1093/jnci/djz009. J Natl Cancer Inst. 2019. PMID: 30753664 Free PMC article. No abstract available.
Similar articles
-
Provider Discussions of Genetic Tests With U.S. Women at Risk for a BRCA Mutation.Am J Prev Med. 2018 Feb;54(2):221-228. doi: 10.1016/j.amepre.2017.10.015. Epub 2017 Dec 11. Am J Prev Med. 2018. PMID: 29241717
-
Screening of over 1000 Indian patients with breast and/or ovarian cancer with a multi-gene panel: prevalence of BRCA1/2 and non-BRCA mutations.Breast Cancer Res Treat. 2018 Jul;170(1):189-196. doi: 10.1007/s10549-018-4726-x. Epub 2018 Feb 22. Breast Cancer Res Treat. 2018. PMID: 29470806
-
BRCA1 and BRCA2 Mutation Testing in Young Women With Breast Cancer.JAMA Oncol. 2016 Jun 1;2(6):730-6. doi: 10.1001/jamaoncol.2015.5941. JAMA Oncol. 2016. PMID: 26867710 Free PMC article.
-
Finding all BRCA pathogenic mutation carriers: best practice models.Eur J Hum Genet. 2016 Sep;24 Suppl 1(Suppl 1):S19-26. doi: 10.1038/ejhg.2016.95. Eur J Hum Genet. 2016. PMID: 27514840 Free PMC article. Review.
-
Towards population-based genetic screenings for breast and ovarian cancer: A comprehensive review from economic evaluations to patient perspectives.Breast. 2021 Aug;58:121-129. doi: 10.1016/j.breast.2021.04.011. Epub 2021 May 12. Breast. 2021. PMID: 34022715 Free PMC article. Review.
Cited by
-
Retrospective assessment of barriers and access to genetic services for hereditary cancer syndromes in an integrated health care delivery system.Hered Cancer Clin Pract. 2022 Feb 10;20(1):7. doi: 10.1186/s13053-022-00213-5. Hered Cancer Clin Pract. 2022. PMID: 35144679 Free PMC article.
-
Psychosocial impact of proactive rapid genetic counseling following breast cancer diagnosis.Psychooncology. 2022 May;31(5):788-797. doi: 10.1002/pon.5863. Epub 2021 Dec 18. Psychooncology. 2022. PMID: 34921700 Free PMC article.
-
Communication about breast cancer genetic counseling with patients with limited health literacy or a migrant background: evaluation of a training program for healthcare professionals.J Community Genet. 2021 Jan;12(1):91-99. doi: 10.1007/s12687-020-00497-x. Epub 2020 Dec 15. J Community Genet. 2021. PMID: 33319336 Free PMC article.
-
PARP inhibition in breast cancer: progress made and future hopes.NPJ Breast Cancer. 2022 Apr 8;8(1):47. doi: 10.1038/s41523-022-00411-3. NPJ Breast Cancer. 2022. PMID: 35396508 Free PMC article.
-
Nationwide Trends and Determinants of Germline BRCA1/2 Testing in Patients With Breast and Ovarian Cancer.J Natl Compr Canc Netw. 2023 Apr;21(4):351-358.e4. doi: 10.6004/jnccn.2022.7257. J Natl Compr Canc Netw. 2023. PMID: 37015340 Free PMC article.
References
-
- Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;91:321–345. - PubMed
-
- Kuchenbaecker KB, Hopper JL, Barnes DR et al. . Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;31723:2402–2416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

