Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings From the VOICE Trial
- PMID: 30753642
- PMCID: PMC6534189
- DOI: 10.1093/infdis/jiz045
Tenofovir Gel for Prevention of Herpes Simplex Virus Type 2 Acquisition: Findings From the VOICE Trial
Abstract
Background: Genital infection with herpes simplex virus type 2 (HSV-2) is common and increases risk of human immunodeficiency virus (HIV) transmission and acquisition. Pericoital use of tenofovir (TFV) gel provided protection from HSV-2 acquisition in the CAPRISA 004 study.
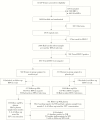
Methods: We measured estimate of effect of vaginal TFV 1% gel in preventing HSV-2 acquisition among women in VOICE, randomized, double-blinded, placebo-controlled trial assessing daily use of oral and vaginal TFV for HIV-1 preexposure prophylaxis. The TFV level in plasma at the first quarterly visit was used as a measure of gel use.
Results: Of 566 participants at risk for HSV-2 acquisition, 532 (94%) had first-quarter plasma TFV and end-of-study HSV-2 serologic data available. Over a follow-up period of 501 person-years, 92 incident cases of HSV-2 acquisition occurred: 77 were in women with no TFV detected in plasma, and 15 occurred in women with TFV detected in plasma (incidence, 20.6 cases/100 person-years [95% confidence interval [CI], 16.2-25.7] vs 11.9 cases/100 person-years [95% CI, 6.6-19.6], respectively). TFV detection in plasma was associated with a trend toward a reduced risk of HSV-2 seroconversion, with an unadjusted hazard ratio (HR) of 0.59 (95% CI, .34-1.02; P = .060) and a HR adjusted for site, age, having ≥2 male sex partners in the past 3 months, use of hormonal contraception, having anal sex in the past 3 months, and HIV status of 0.60 (95% CI, .33-1.08; P = .086).
Conclusions: Detection of TFV in plasma among TFV gel users was associated with a trend toward a reduced risk of HSV-2 acquisition, after controlling for sexual behavior and HIV-1 acquisition.
Keywords: HIV-1; Herpes simplex virus; genital herpes; preexposure prophylaxis; tenofovir.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Similar articles
-
The Vaginal Acquisition and Dissemination of HIV-1 Infection in a Novel Transgenic Mouse Model Is Facilitated by Coinfection with Herpes Simplex Virus 2 and Is Inhibited by Microbicide Treatment.J Virol. 2015 Sep;89(18):9559-70. doi: 10.1128/JVI.01326-15. Epub 2015 Jul 8. J Virol. 2015. PMID: 26157126 Free PMC article.
-
Oral and Vaginal Tenofovir for Genital Herpes Simplex Virus Type 2 Shedding in Immunocompetent Women: A Double-Blind, Randomized, Cross-over Trial.J Infect Dis. 2015 Dec 15;212(12):1949-56. doi: 10.1093/infdis/jiv317. Epub 2015 Jun 4. J Infect Dis. 2015. PMID: 26044291 Free PMC article. Clinical Trial.
-
Daily oral tenofovir and emtricitabine-tenofovir preexposure prophylaxis reduces herpes simplex virus type 2 acquisition among heterosexual HIV-1-uninfected men and women: a subgroup analysis of a randomized trial.Ann Intern Med. 2014 Jul 1;161(1):11-9. doi: 10.7326/M13-2471. Ann Intern Med. 2014. PMID: 24979446 Clinical Trial.
-
Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection.J Infect Dis. 2005 Feb 1;191 Suppl 1:S107-14. doi: 10.1086/425272. J Infect Dis. 2005. PMID: 15627220 Review.
-
The Vaginal Microbiome and its Potential to Impact Efficacy of HIV Pre-exposure Prophylaxis for Women.Curr HIV/AIDS Rep. 2017 Oct;14(5):153-160. doi: 10.1007/s11904-017-0362-z. Curr HIV/AIDS Rep. 2017. PMID: 28812207 Free PMC article. Review.
Cited by
-
Multipurpose Prevention Technologies: Oral, Parenteral, and Vaginal Dosage Forms for Prevention of HIV/STIs and Unplanned Pregnancy.Polymers (Basel). 2021 Jul 26;13(15):2450. doi: 10.3390/polym13152450. Polymers (Basel). 2021. PMID: 34372059 Free PMC article. Review.
-
Subclinical Genital Herpes Shedding in HIV/Herpes Simplex Virus 2-Coinfected Women during Antiretroviral Therapy Is Associated with an Increase in HIV Tissue Reservoirs and Potentially Promotes HIV Evolution.J Virol. 2020 Dec 9;95(1):e01606-20. doi: 10.1128/JVI.01606-20. Print 2020 Dec 9. J Virol. 2020. PMID: 33028713 Free PMC article.
-
Where are the pregnant and breastfeeding women in new pre-exposure prophylaxis trials? The imperative to overcome the evidence gap.Lancet HIV. 2022 Mar;9(3):e214-e222. doi: 10.1016/S2352-3018(21)00280-0. Epub 2022 Jan 25. Lancet HIV. 2022. PMID: 35090604 Free PMC article. Review.
-
Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection.Pharmaceuticals (Basel). 2021 May 11;14(5):454. doi: 10.3390/ph14050454. Pharmaceuticals (Basel). 2021. PMID: 34064831 Free PMC article. Review.
-
Single dose topical inserts containing tenofovir alafenamide fumarate and elvitegravir provide pre- and post-exposure protection against vaginal SHIV infection in macaques.EBioMedicine. 2022 Dec;86:104361. doi: 10.1016/j.ebiom.2022.104361. Epub 2022 Nov 21. EBioMedicine. 2022. PMID: 36423375 Free PMC article.
References
-
- Bradley J, Floyd S, Piwowar-Manning E, et al. ; HPTN 071 (PopART) Study Team Sexually transmitted bedfellows: exquisite association between hiv and herpes simplex virus type 2 in 21 communities in Southern Africa in the HIV prevention trials network 071 (PopART) study. J Infect Dis 2018; 218:443–52. - PMC - PubMed
-
- Andrei G, Gillemot S, Topalis D, Snoeck R. The anti-human immunodeficiency virus drug tenofovir, a reverse transcriptase inhibitor, also targets the herpes simplex virus DNA polymerase. J Infect Dis 2018; 217:790–801. - PubMed

