Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and BrafV600E-Induced Tumorigenesis
- PMID: 30753828
- PMCID: PMC6636642
- DOI: 10.1016/j.ccell.2019.01.005
Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and BrafV600E-Induced Tumorigenesis
Abstract
We addressed the precursor role of aging-like spontaneous promoter DNA hypermethylation in initiating tumorigenesis. Using mouse colon-derived organoids, we show that promoter hypermethylation spontaneously arises in cells mimicking the human aging-like phenotype. The silenced genes activate the Wnt pathway, causing a stem-like state and differentiation defects. These changes render aged organoids profoundly more sensitive than young ones to transformation by BrafV600E, producing the typical human proximal BRAFV600E-driven colon adenocarcinomas characterized by extensive, abnormal gene-promoter CpG-island methylation, or the methylator phenotype (CIMP). Conversely, CRISPR-mediated simultaneous inactivation of a panel of the silenced genes markedly sensitizes to BrafV600E-induced transformation. Our studies tightly link aging-like epigenetic abnormalities to intestinal cell fate changes and predisposition to oncogene-driven colon tumorigenesis.
Keywords: BRAF(V600E); CIMP; CpG-island DNA methylation; aging; cancer risk; colon adenocarcinomas; epigenetic silencing; transformation; tumor predisposition; tumorigenesis.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures





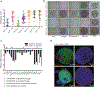
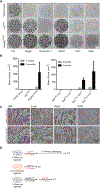
Comment in
-
The Origin of CIMP, At Last.Cancer Cell. 2019 Feb 11;35(2):165-167. doi: 10.1016/j.ccell.2019.01.015. Cancer Cell. 2019. PMID: 30753821
References
-
- Ahuja N, Li Q, Mohan AL, Baylin SB, and Issa JP (1998). Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 58, 5489–5494. - PubMed
-
- Akalin A, Franke V, Vlahovicek K, Mason CE, and Schubeler D (2015). Genomation: a toolkit to summarize, annotate and visualize genomic intervals. Bioinformatics 31, 1127–1129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

