Dysregulated gene expression in oocysts of Plasmodium berghei LAP mutants
- PMID: 30753856
- PMCID: PMC6452916
- DOI: 10.1016/j.molbiopara.2019.02.001
Dysregulated gene expression in oocysts of Plasmodium berghei LAP mutants
Abstract
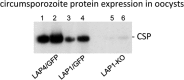
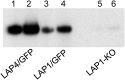
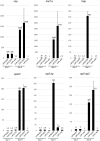
Malaria parasite oocysts generate sporozoites by a process termed sporogony. Essential for successful sporogony of Plasmodium berghei in Anopheles stephensi mosquitoes is a complex of six LCCL lectin domain adhesive-like proteins (LAPs). LAP null mutant oocysts undergo growth and mitosis but fail to form sporozoites. At a cytological level, LAP null mutant oocyst development is indistinguishable from its wildtype counterparts for the first week, supporting the hypothesis that LAP null mutant oocysts develop normally before cytokinesis. We show here that LAP1 null mutant oocysts display highly reduced expression of sporozoite proteins and their transcription factors. Our findings indicate that events leading up to the cytokinesis defect in LAP null mutants occur early in oocyst development.
Keywords: LCCL; Malaria transmission; Oocyst; Plasmodium berghei; Sporogony.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
The Plasmodium LAP complex affects crystalloid biogenesis and oocyst cell division.Int J Parasitol. 2018 Dec;48(14):1073-1078. doi: 10.1016/j.ijpara.2018.09.002. Epub 2018 Oct 24. Int J Parasitol. 2018. PMID: 30367865 Free PMC article.
-
Plasmodium berghei sporozoite specific genes- PbS10 and PbS23/SSP3 are required for the development of exo-erythrocytic forms.Mol Biochem Parasitol. 2019 Sep;232:111198. doi: 10.1016/j.molbiopara.2019.111198. Epub 2019 Jun 26. Mol Biochem Parasitol. 2019. PMID: 31251952
-
Plasmodium berghei Cap93, a novel oocyst capsule-associated protein, plays a role in sporozoite development.Parasit Vectors. 2017 Aug 25;10(1):399. doi: 10.1186/s13071-017-2337-8. Parasit Vectors. 2017. PMID: 28841886 Free PMC article.
-
Protein O-Fucosyltransferase 2 Is Not Essential for Plasmodium berghei Development.Front Cell Infect Microbiol. 2019 Jul 3;9:238. doi: 10.3389/fcimb.2019.00238. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31334132 Free PMC article.
-
Crystalloids: Fascinating Parasite Organelles Essential for Malaria Transmission.Trends Parasitol. 2021 Jul;37(7):581-584. doi: 10.1016/j.pt.2021.04.002. Epub 2021 Apr 30. Trends Parasitol. 2021. PMID: 33941493 Review.
Cited by
-
The Multiple Roles of LCCL Domain-Containing Proteins for Malaria Parasite Transmission.Microorganisms. 2024 Jan 29;12(2):279. doi: 10.3390/microorganisms12020279. Microorganisms. 2024. PMID: 38399683 Free PMC article. Review.
-
Who Needs a Contractile Actomyosin Ring? The Plethora of Alternative Ways to Divide a Protozoan Parasite.Front Cell Infect Microbiol. 2019 Nov 21;9:397. doi: 10.3389/fcimb.2019.00397. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31824870 Free PMC article. Review.
-
Plasmodium berghei oocysts possess fatty acid synthesis and scavenging routes.Sci Rep. 2023 Aug 5;13(1):12700. doi: 10.1038/s41598-023-39708-z. Sci Rep. 2023. PMID: 37543672 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

