Microfluidic High-Migratory Cell Collector Suppressing Artifacts Caused by Microstructures
- PMID: 30754704
- PMCID: PMC6412487
- DOI: 10.3390/mi10020116
Microfluidic High-Migratory Cell Collector Suppressing Artifacts Caused by Microstructures
Abstract
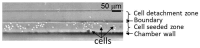
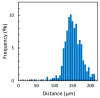
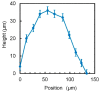
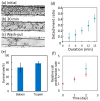
The small number of high-migratory cancer cells in a cell population make studies on high-migratory cancer cells difficult. For the development of migration assays for such cancer cells, several microfluidic devices have been developed. However, they measure migration that is influenced by microstructures and they collect not only high-migratory cells, but also surrounding cells. In order to find high-migratory cells in cell populations while suppressing artifacts and to collect these cells while minimizing damages, we developed a microfluidic high-migratory cell collector with the ability to sort cancer cells according to cellular migration and mechanical detachment. High-migratory cancer cells travel further from the starting line when all of the cells are seeded on the same starting line. The high-migratory cells are detached using a stretch of cell adhesive surface using a water-driven balloon actuator. Using this cell collector, we selected high-migratory HeLa cells that migrated about 100m in 12 h and collected the cells.
Keywords: balloon; high-migratory cell; microfluidic cell collector; migration assay.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures






Similar articles
-
A versatile cancer cell trapping and 1D migration assay in a microfluidic device.Biomicrofluidics. 2019 Jul 23;13(4):044105. doi: 10.1063/1.5103269. eCollection 2019 Jul. Biomicrofluidics. 2019. PMID: 31372193 Free PMC article.
-
Cell migration microfluidics for electrotaxis-based heterogeneity study of lung cancer cells.Biosens Bioelectron. 2017 Mar 15;89(Pt 2):837-845. doi: 10.1016/j.bios.2016.10.002. Epub 2016 Oct 4. Biosens Bioelectron. 2017. PMID: 27816579
-
Functional Isolation of Tumor-Initiating Cells using Microfluidic-Based Migration Identifies Phosphatidylserine Decarboxylase as a Key Regulator.Sci Rep. 2018 Jan 10;8(1):244. doi: 10.1038/s41598-017-18610-5. Sci Rep. 2018. PMID: 29321615 Free PMC article.
-
Cell Migration in Microfluidic Devices: Invadosomes Formation in Confined Environments.Adv Exp Med Biol. 2019;1146:79-103. doi: 10.1007/978-3-030-17593-1_6. Adv Exp Med Biol. 2019. PMID: 31612455 Review.
-
Microfluidic Lab-on-a-Chip for Studies of Cell Migration under Spatial Confinement.Biosensors (Basel). 2022 Aug 5;12(8):604. doi: 10.3390/bios12080604. Biosensors (Basel). 2022. PMID: 36004998 Free PMC article. Review.
Cited by
-
Microvalve with Trapezoid-Shaped Cross-Section for Deep Microchannels.Micromachines (Basel). 2021 Nov 15;12(11):1403. doi: 10.3390/mi12111403. Micromachines (Basel). 2021. PMID: 34832813 Free PMC article.
-
Cell Culture on Low-Fluorescence and High-Resolution Photoresist.Micromachines (Basel). 2020 Jun 4;11(6):571. doi: 10.3390/mi11060571. Micromachines (Basel). 2020. PMID: 32512915 Free PMC article.
-
Portable Rice Disease Spores Capture and Detection Method Using Diffraction Fingerprints on Microfluidic Chip.Micromachines (Basel). 2019 Apr 28;10(5):289. doi: 10.3390/mi10050289. Micromachines (Basel). 2019. PMID: 31035416 Free PMC article.
References
-
- Ishida T., Shimamoto T., Ozaki N., Takaki S., Kuchimaru T., Kizaka-Kondoh S., Omata T. Investigation of The Influence of Glucose Concentration on Cancer Cells by Using a Microfluidic Gradient Generator without the Induction of Large Shear Stress. Micromachines. 2016;7:155. doi: 10.3390/mi7090155. - DOI - PMC - PubMed
-
- Martin T.A., Ye L., Sanders A.J., Lane J., Jiang W.G. Cancer Invasion and Metastasis: Molecular and Cellular Perspective. Landes Bioscience; Austin, TX, USA: 2013.
Grants and funding
LinkOut - more resources
Full Text Sources

