Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect
- PMID: 30754707
- PMCID: PMC6406319
- DOI: 10.3390/jcm8020234
Trastuzumab Induced Chemobrain, Atorvastatin Rescued Chemobrain with Enhanced Anticancer Effect and without Hair Loss-Side Effect
Abstract
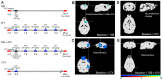
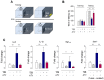
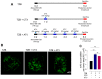
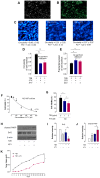
The authors identified that chemo-brain was induced after trastuzumab (TZB) therapy. In addition, atorvastatin (ATV) could rescue chemo-brain during trastuzumab (TZB) therapy. Enhanced therapeutic effect of TZB was confirmed after ATV therapy. We also investigated that there was no hair loss side effect due to ATV therapy. In an animal model, 150 μg TZB and five serial doses of 20 mg/kg ATV were administered. 18F-fluorodeoxyglucose Positron Emission Tomography (PET) and Magnetic Resonance Imaging (MRI) data were acquired. Statistical parametric mapping analysis and voxel-based morphometry analysis were performed to identify differences in glucose metabolism and gray matter concentration. The enhanced therapeutic efficacy of TZB after ATV treatment was assessed using a human epidermal growth factor receptor 2-positive gastric cancer model. We found a decrease in cerebral glucose metabolism and gray matter concentration in the frontal lobe following TZB therapy (p < 0.005). After subsequent ATV administration, glucose metabolism and regional gray matter concentration were rescued (p < 0.005). Cognitive impairment due to TZB and the rescue effect of ATV were confirmed using a passive avoidance test and quantitative real-time reverse transcription PCR. Furthermore, the penetration and accumulation of TZB in tumors increased by 100% after ATV co-administration, which resulted in an enhanced anti-cancer effect. Our study collectively demonstrates that ATV co-administration with TZB rescued the TZB-induced chemo-brain and enhances the therapeutic efficacy of TZB in tumors. We also showed that there was no hair loss during ATV therapy.
Keywords: anti-cancer effect; atorvastatin; chemo-brain; radiomics; trastuzumab therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

