Removal of Paracetamol Using Effective Advanced Oxidation Processes
- PMID: 30754732
- PMCID: PMC6388171
- DOI: 10.3390/ijerph16030505
Removal of Paracetamol Using Effective Advanced Oxidation Processes
Abstract
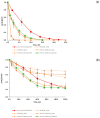
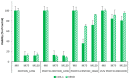
Fenton, photo-Fenton, and photo-induced oxidation, were investigated and compared for the treatment of 0.26 mmol L-1 of paracetamol (PCT) in a deionised water matrix, during a reaction span of 120.0 min. Low and high Fenton reagent loads were studied. Particularly, the initial concentration of Fe2+ was varied between 0.09 and 0.18 mmol L-1 while the initial concentration of H₂O₂ was varied between 2.78 and 11.12 mmol L-1. The quantitative performance of these treatments was evaluated by: (i) measuring PCT concentration; (ii) measuring and modelling TOC conversion, as a means characterizing sample mineralization; and (iii) measuring cytotoxicity to assess the safe application of each treatment. In all cases, organic matter mineralization was always partial, but PCT concentration fell below the detection limit within 2.5 and 20.0 min. The adopted semi-empirical model revealed that photo induced oxidation is the only treatment attaining total organic matter mineralization ( ξ MAX = 100% in 200.0 min) at the expense of the lowest kinetic constant (k = 0.007 min-1). Conversely, photo-Fenton treatment using high Fenton reagent loads gave a compromise solution ( ξ MAX = 73% and k = 0.032 min-1). Finally, cytotoxicity assays proved the safe application of photo-induced oxidation and of photo-Fenton treatments using high concentrations of Fenton reagents.
Keywords: Fenton; by-products; cytotoxicity; hydrogen peroxide dosage; kinetic model; paracetamol; photo-Fenton; photo-induced oxidation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ternes T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998;32:3245–3260. doi: 10.1016/S0043-1354(98)00099-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

