Cellular immunotherapy in multiple myeloma
- PMID: 30754964
- PMCID: PMC6718748
- DOI: 10.3904/kjim.2018.325
Cellular immunotherapy in multiple myeloma
Abstract
In multiple myeloma (MM), the impaired function of several types of immune cells favors the tumor's escape from immune surveillance and, therefore, its growth and survival. Tremendous improvements have been made in the treatment of MM over the past decade but cellular immunotherapy using dendritic cells, natural killer cells, and genetically engineered T-cells represent a new therapeutic era. The application of these treatments is growing rapidly, based on their capacity to eradicate MM. In this review, we summarize recent progress in cellular immunotherapy for MM and its future prospects.
Keywords: Cellular immunotherapy; Dendritic cells; Engineered effector T cell; Immunomodulatory drug; Killer cells, natural; Multiple myeloma.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



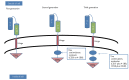
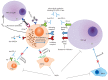
References
-
- Sirohi B, Powles R. Multiple myeloma. Lancet. 2004;363:875–887. - PubMed
-
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. - PubMed
-
- Lonial S, Cavenagh J. Emerging combination treatment strategies containing novel agents in newly diagnosed multiple myeloma. Br J Haematol. 2009;145:681–708. - PubMed
-
- Attal M, Harousseau JL. The role of high-dose therapy with autologous stem cell support in the era of novel agents. Semin Hematol. 2009;46:127–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
