Inositol 1,4,5-Trisphosphate Receptors in Endothelial Cells Play an Essential Role in Vasodilation and Blood Pressure Regulation
- PMID: 30755057
- PMCID: PMC6405661
- DOI: 10.1161/JAHA.118.011704
Inositol 1,4,5-Trisphosphate Receptors in Endothelial Cells Play an Essential Role in Vasodilation and Blood Pressure Regulation
Abstract
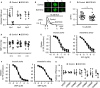
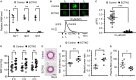
Background Endothelial NO synthase plays a central role in regulating vasodilation and blood pressure. Intracellular Ca2+ mobilization is a critical modulator of endothelial NO synthase function, and increased cytosolic Ca2+ concentration in endothelial cells is able to induce endothelial NO synthase phosphorylation. Ca2+ release mediated by 3 subtypes of inositol 1,4,5-trisphosphate receptors ( IP 3Rs) from the endoplasmic reticulum and subsequent Ca2+ entry after endoplasmic reticulum Ca2+ store depletion has been proposed to be the major pathway to mobilize Ca2+ in endothelial cells. However, the physiological role of IP 3Rs in regulating blood pressure remains largely unclear. Methods and Results To investigate the role of endothelial IP 3Rs in blood pressure regulation, we first generated an inducible endothelial cell-specific IP 3R1 knockout mouse model and found that deletion of IP 3R1 in adult endothelial cells did not affect vasodilation and blood pressure. Considering all 3 subtypes of IP 3Rs are expressed in mouse endothelial cells, we further generated inducible endothelial cell-specific IP 3R triple knockout mice and found that deletion of all 3 IP 3R subtypes decreased plasma NO concentration and increased basal blood pressure. Furthermore, IP 3R deficiency reduced acetylcholine-induced vasodilation and endothelial NO synthase phosphorylation at Ser1177. Conclusions Our results reveal that IP 3R-mediated Ca2+ release in vascular endothelial cells plays an important role in regulating vasodilation and physiological blood pressure.
Keywords: blood pressure; calcium; calcium signaling; endothelial cell; hypertension.
Figures


References
-
- Blacher J, Levy BI, Mourad JJ, Safar ME, Bakris G. From epidemiological transition to modern cardiovascular epidemiology: hypertension in the 21st century. Lancet. 2016;388:530–532. - PubMed
-
- Kokubo Y, Iwashima Y. Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension. 2015;66:254–259. - PubMed
-
- Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short‐term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. - PubMed
-
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L‐arginine. Nature. 1988;333:664–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

