Relationship Between Low-Density Lipoprotein Cholesterol and Lipoprotein(a) Lowering in Response to PCSK9 Inhibition With Evolocumab
- PMID: 30755061
- PMCID: PMC6405654
- DOI: 10.1161/JAHA.118.010932
Relationship Between Low-Density Lipoprotein Cholesterol and Lipoprotein(a) Lowering in Response to PCSK9 Inhibition With Evolocumab
Abstract
Background Beyond their potent LDL (low-density lipoprotein) cholesterol ( LDL -C)-lowering efficacy (50-60%), PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitors also reduce Lp(a) (lipoprotein[a]) levels by 25% to 30%, suggesting a 2:1 response ratio. We aimed to characterize the relationship between LDL -C and Lp(a) lowering by evolocumab, a PCSK 9 inhibitor, in a large clinical trial population and to determine the prevalence of concordant/discordant LDL -C and Lp(a) responses to PCSK 9 inhibition. Methods and Results Data were analyzed from 4 randomized, 12-week, multicenter, phase 3 evolocumab trials. Patients with familial hypercholesterolemia, nonfamilial hypercholesterolemia, or statin intolerance participated in the trials. The main measure was the degree of concordance or discordance of LDL -C and Lp(a) in response to PCSK 9 inhibition; concordant response was defined as LDL -C reduction >35% and Lp(a) reduction >10%. The study cohort comprised 895 patients (438 female; median age: 59.0 years [interquartile range: 51-66 years]). Baseline mean level of LDL -C was 133.6 mg/dL (SE: 1.7) and median Lp(a) level was 46.4 mg/dL (interquartile range: 18.4-82.4 mg/dL). A discordant response was observed in 165 (19.7%) patients. With these cutoffs, the prevalence of discordance was higher when considering baseline Lp(a) concentrations >30 mg/dL (26.5%) or >50 mg/dL (28.6%). Conclusions We demonstrate high prevalence of discordance in LDL -C and Lp(a) reduction in response to evolocumab, particularly when considering higher baseline Lp(a) concentrations, indicating the possibility of alternative pathways beyond LDLR ( LDL receptor)-mediated clearance involved in Lp(a) reduction by evolocumab. Clinical Trial Registration URL : http://www.clinicaltrials.gov . Unique identifiers: NCT 01763827, NCT 01763866, NCT 01763905, NCT 01763918.
Trial registration: ClinicalTrials.gov NCT01763827 NCT01763866 NCT01763905 NCT01763918.
Keywords: lipid‐lowering therapy; lipoprotein[a]; low‐density lipoprotein cholesterol; proprotein convertase subtilisin/kexin type 9.
Figures
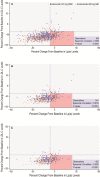
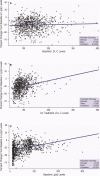
Comment in
-
Lipoprotein(a) Removal Still a Mystery.J Am Heart Assoc. 2019 Feb 19;8(4):e011903. doi: 10.1161/JAHA.118.011903. J Am Heart Assoc. 2019. PMID: 30755058 Free PMC article.
Similar articles
-
Discordant responses of plasma low-density lipoprotein cholesterol and lipoprotein(a) to alirocumab: A pooled analysis from 10 ODYSSEY Phase 3 studies.Eur J Prev Cardiol. 2021 Jul 23;28(8):816-822. doi: 10.1177/2047487320915803. Epub 2020 Apr 10. Eur J Prev Cardiol. 2021. PMID: 34298554
-
Association Between Circulating Baseline Proprotein Convertase Subtilisin Kexin Type 9 Levels and Efficacy of Evolocumab.JAMA Cardiol. 2017 May 1;2(5):556-560. doi: 10.1001/jamacardio.2016.5395. JAMA Cardiol. 2017. PMID: 28122070 Free PMC article.
-
Lipoprotein(a), PCSK9 Inhibition, and Cardiovascular Risk.Circulation. 2019 Mar 19;139(12):1483-1492. doi: 10.1161/CIRCULATIONAHA.118.037184. Circulation. 2019. PMID: 30586750 Clinical Trial.
-
Management of Hypercholesterolemia, Appropriateness of Therapeutic Approaches and New Drugs in Patients with High Cardiovascular Risk.High Blood Press Cardiovasc Prev. 2016 Sep;23(3):217-30. doi: 10.1007/s40292-016-0155-2. Epub 2016 Aug 27. High Blood Press Cardiovasc Prev. 2016. PMID: 27567901 Free PMC article. Review.
-
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: Present perspectives and future horizons.Nutr Metab Cardiovasc Dis. 2016 Oct;26(10):853-62. doi: 10.1016/j.numecd.2016.05.006. Epub 2016 May 30. Nutr Metab Cardiovasc Dis. 2016. PMID: 27352986 Review.
Cited by
-
Proprotein convertase subtilisin/kexin type 9 inhibition in cardiovascular disease: current status and future perspectives.Korean J Intern Med. 2020 Sep;35(5):1045-1058. doi: 10.3904/kjim.2020.140. Epub 2020 Aug 28. Korean J Intern Med. 2020. PMID: 32921006 Free PMC article. Review.
-
ANGPLT3 in cardio-metabolic disorders.Mol Biol Rep. 2021 Mar;48(3):2729-2739. doi: 10.1007/s11033-021-06248-6. Epub 2021 Mar 6. Mol Biol Rep. 2021. PMID: 33677817 Review.
-
Comprehensive Investigation of Circulating Biomarkers and Their Causal Role in Atherosclerosis-Related Risk Factors and Clinical Events.Circ Genom Precis Med. 2020 Dec;13(6):e002996. doi: 10.1161/CIRCGEN.120.002996. Epub 2020 Oct 30. Circ Genom Precis Med. 2020. PMID: 33125266 Free PMC article.
-
Low circulating PCSK9 levels in LPL homozygous children with chylomicronemia syndrome in a syrian refugee family in Lebanon.Front Genet. 2022 Aug 19;13:961028. doi: 10.3389/fgene.2022.961028. eCollection 2022. Front Genet. 2022. PMID: 36061186 Free PMC article.
-
Effectiveness of proprotein convertase subtilisin/kexin-9 monoclonal antibody treatment on plasma lipoprotein(a) concentrations in patients with elevated lipoprotein(a) attending a clinic.Clin Cardiol. 2021 Jun;44(6):805-813. doi: 10.1002/clc.23607. Epub 2021 May 6. Clin Cardiol. 2021. PMID: 33955565 Free PMC article.
References
-
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. - PubMed
-
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM; Committees OO, Investigators . Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. - PubMed
-
- Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H; MENDEL‐2 Investigators . Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. - PubMed
-
- Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni‐Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D; RUTHERFORD‐2 Investigators . PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

