Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis
- PMID: 30755068
- PMCID: PMC6435921
- DOI: 10.1177/1098612X19825701
Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis
Abstract
Objectives: The aim of this study was to determine the safety and efficacy of the nucleoside analog GS-441524 for cats suffering from various forms of naturally acquired feline infectious peritonitis (FIP).
Methods: Cats ranged from 3.4-73 months of age (mean 13.6 months); 26 had effusive or dry-to-effusive FIP and five had non-effusive disease. Cats with severe neurological and ocular FIP were not recruited. The group was started on GS-441524 at a dosage of 2.0 mg/kg SC q24h for at least 12 weeks and increased when indicated to 4.0 mg/kg SC q24h.
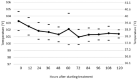
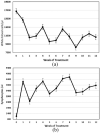
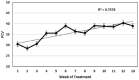
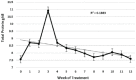
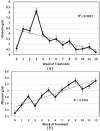
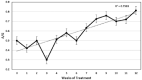
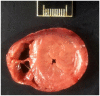
Results: Four of the 31 cats that presented with severe disease died or were euthanized within 2-5 days and a fifth cat after 26 days. The 26 remaining cats completed the planned 12 weeks or more of treatment. Eighteen of these 26 cats remain healthy at the time of publication (OnlineFirst, February 2019) after one round of treatment, while eight others suffered disease relapses within 3-84 days. Six of the relapses were non-neurological and two neurological. Three of the eight relapsing cats were treated again at the same dosage, while five cats had the dosage increased from 2.0 to 4.0 mg/kg q24h. The five cats treated a second time at the higher dosage, including one with neurological disease, responded well and also remain healthy at the time of publication. However, one of the three cats re-treated at the original lower dosage relapsed with neurological disease and was euthanized, while the two remaining cats responded favorably but relapsed a second time. These two cats were successfully treated a third time at the higher dosage, producing 25 long-time survivors. One of the 25 successfully treated cats was subsequently euthanized due to presumably unrelated heart disease, while 24 remain healthy.
Conclusions and relevance: GS-441524 was shown to be a safe and effective treatment for FIP. The optimum dosage was found to be 4.0 mg/kg SC q24h for at least 12 weeks.
Keywords: FIP; GS-441524; Nucleoside analog; feline infectious peritonitis; field trial.
Conflict of interest statement
MP and EM are employees of Gilead Sciences, Foster City, CA, USA, and hold stock interests in the company.
Figures

References
-
- Thomasy SM, Shull O, Outerbridge CA, et al. Oral administration of famciclovir for treatment of spontaneous ocular, respiratory, or dermatologic disease attributed to feline herpesvirus type 1: 59 cases (2006–2013). J Am Vet Med Assoc 2016; 249: 526–538. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

