β-Blockers and 1-Year Postdischarge Mortality for Heart Failure and Reduced Ejection Fraction and Slow Discharge Heart Rate
- PMID: 30755071
- PMCID: PMC6405672
- DOI: 10.1161/JAHA.118.011121
β-Blockers and 1-Year Postdischarge Mortality for Heart Failure and Reduced Ejection Fraction and Slow Discharge Heart Rate
Abstract
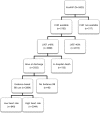
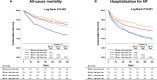
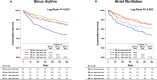
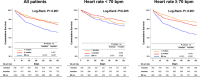
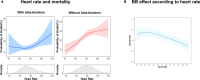
Background Many hospitalized patients with heart failure and reduced ejection fraction ( HF r EF ) have a slow heart rate at discharge, and the effect of β-blockers may be reduced in those patients. We sought to examine the variable effect of β-blockers on clinical outcomes according to the discharge heart rate of hospitalized HF r EF patients. Methods and Results The KorAHF (Korean Acute Heart Failure) registry consecutively enrolled 5625 patients hospitalized for acute heart failure. In this analysis, we included patients with HF r EF (left ventricular ejection fraction ≤40%). Slow heart rate was defined as <70 beats per minute regardless of the use of β-blockers. The primary outcome was 1-year all-cause postdischarge death according to heart rate. Among 2932 patients with HF r EF , 840 (29%) had a slow heart rate and 56% received β-blockers at discharge. Patients with slow heart rates were older and had lower 1-year mortality than those with high heart rates ( P<0.001). A significant interaction between discharge heart rate and β-blocker use was observed ( P<0.001 for interaction). When stratified, only patients without a β-blocker prescription and with a high heart rate showed higher 1-year mortality. In a Cox-proportional hazards regression analysis, β-blocker prescription at discharge was associated with 24% reduced risk for 1-year mortality in patients with high heart rates (hazard ratio: 0.76; 95% CI, 0.61-0.95) but not in those with slow heart rates (hazard ratio: 1.02; 95% CI, 0.68-1.55). Conclusions Many patients with acute heart failure have slow discharge heart rates, and β-blockers may have a limited effect on HF r EF and slow discharge heart rate. Clinical Trial Registration URL : http://www.clinicaltrial.gov . Unique identifier: NCT 01389843.
Trial registration: ClinicalTrials.gov NCT01389843.
Keywords: heart failure; heart rate; outcome; β‐blocker.
Figures

References
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. - PubMed
-
- Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. - PubMed
-
- Kjekshus J, Gullestad L. Heart rate as a therapeutic target in heart failure. Eur Heart J Suppl. 1999;1:64–69.
-
- Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta‐blockers in heart failure: findings from the OPTIMIZE‐HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53:184–192. - PMC - PubMed
-
- Gullestad L, Wikstrand J, Deedwania P, Hjalmarson A, Egstrup K, Elkayam U, Gottlieb S, Rashkow A, Wedel H, Bermann G, Kjekshus J; MERIT‐HF Study Group . What resting heart rate should one aim for when treating patients with heart failure with a beta‐blocker? Experiences from the Metoprolol Controlled Release/Extended Release Randomized Intervention Trial in Chronic Heart Failure (MERIT‐HF). J Am Coll Cardiol. 2005;45:252–259. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

