Comparison of HepaRG cells following growth in proliferative and differentiated culture conditions reveals distinct bioenergetic profiles
- PMID: 30755072
- PMCID: PMC6422474
- DOI: 10.1080/15384101.2019.1578133
Comparison of HepaRG cells following growth in proliferative and differentiated culture conditions reveals distinct bioenergetic profiles
Abstract
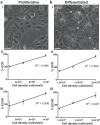
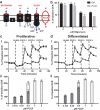
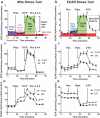
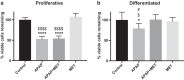
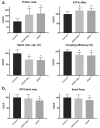
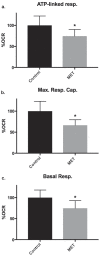
HepaRG is a proliferative human hepatoma-derived cell line that can be differentiated into hepatocyte-like and biliary-like cells. Differentiated HepaRG cultures maintain key hepatic functions including drug transporters and xenobiotic-metabolizing enzymes. To gain insight into proliferative and differentiated HepaRG metabolism we profiled various bioenergetic parameters and investigated cell culture levels of adenosine triphosphate (ATP), lactate, and lactate dehydrogenase (LDH) activity. Compared to differentiated-derived HepaRG, cells from proliferative cultures had increased basal and ATP-linked respiration and decreased maximal and spare respiratory capacities. Basal ATP levels but not lactate or LDH activity were increased in samples from proliferative-derived compared to differentiated-derived HepaRG. Further extracellular acidification rate (ECAR) experiments revealed parameters associated with glycolysis and oxidative phosphorylation. Under basal conditions, cells derived from both cultures had similar ECARs; however, under stressed conditions, proliferative-derived HepaRG had increases in ECAR capacity and apparent glycolytic reserve. The biguanide metformin has been reported to protect differentiated HepaRG against acetaminophen (APAP)-induced cell injury, as well as offer protection against bioenergetic deficiencies; therefore, we studied the outcome of exposure to these drugs in both culture conditions. Proliferative- and differentiated-derived cells were found to have distinct mitochondrial bioenergetic alterations when exposed to the hepatotoxic drug APAP. Metformin offered protection against loss of APAP-induced cellular viability and prevented APAP-induced decreases in bioenergetics in differentiated- but not proliferative-derived HepaRG. Distinguishingly, treatment with metformin alone reduced ATP-linked respiration, maximal respiratory capacity, and basal respiration in proliferative-derived HepaRG. Our results support that HepaRG represents an appropriate model to study drug-induced bioenergetic dysfunction.
Keywords: Extracellular flux analysis and bioenergetics; pharmacological stressors; proliferative and differentiated HepaRG.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
