Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles
- PMID: 30755300
- PMCID: PMC6400823
- DOI: 10.1016/j.bpj.2019.01.016
Gramicidin Increases Lipid Flip-Flop in Symmetric and Asymmetric Lipid Vesicles
Abstract
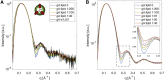
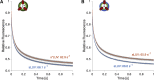
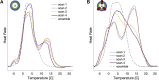
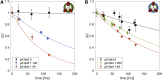
Unlike most transmembrane proteins, phospholipids can migrate from one leaflet of the membrane to the other. Because this spontaneous lipid translocation (flip-flop) tends to be very slow, cells facilitate the process with enzymes that catalyze the transmembrane movement and thereby regulate the transbilayer lipid distribution. Nonenzymatic membrane-spanning proteins with unrelated primary functions have also been found to accelerate lipid flip-flop in a nonspecific manner and by various hypothesized mechanisms. Using deuterated phospholipids, we examined the acceleration of flip-flop by gramicidin channels, which have well-defined structures and known functions, features that make them ideal candidates for probing the protein-membrane interactions underlying lipid flip-flop. To study compositionally and isotopically asymmetric proteoliposomes containing gramicidin, we expanded a recently developed protocol for the preparation and characterization of lipid-only asymmetric vesicles. Channel incorporation, conformation, and function were examined with small angle x-ray scattering, circular dichroism, and a stopped-flow spectrofluorometric assay, respectively. As a measure of lipid scrambling, we used differential scanning calorimetry to monitor the effect of gramicidin on the melting transition temperatures of the two bilayer leaflets. The two calorimetric peaks of the individual leaflets merged into a single peak over time, suggestive of scrambling, and the effect of the channel on the transbilayer lipid distribution in both symmetric 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and asymmetric 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine/1,2-dimyristoyl-sn-glycero-3-phosphocholine vesicles was quantified from proton NMR measurements. Our results show that gramicidin increases lipid flip-flop in a complex, concentration-dependent manner. To determine the molecular mechanism of the process, we used molecular dynamics simulations and further computational analysis of the trajectories to estimate the extent of membrane deformation. Together, the experimental and computational approaches were found to constitute an effective means for studying the effects of transmembrane proteins on lipid distribution in both symmetric and asymmetric model membranes.
Copyright © 2019 Biophysical Society. All rights reserved.
Figures
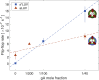

References
-
- Perillo V.L., Peñalva D.A., Antollini S.S. Transbilayer asymmetry and sphingomyelin composition modulate the preferential membrane partitioning of the nicotinic acetylcholine receptor in Lo domains. Arch. Biochem. Biophys. 2016;591:76–86. - PubMed
-
- Perlmutter J.D., Sachs J.N. Interleaflet interaction and asymmetry in phase separated lipid bilayers: molecular dynamics simulations. J. Am. Chem. Soc. 2011;133:6563–6577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

