Bone Metastasis: Find Your Niche and Fit in
- PMID: 30755309
- PMCID: PMC6383208
- DOI: 10.1016/j.trecan.2018.12.004
Bone Metastasis: Find Your Niche and Fit in
Abstract
Metastasis to bones is determined by both intrinsic traits of metastatic tumor cells and properties appertaining to the bone microenvironment. Bone marrow niches are critical for all major steps of metastasis, including the seeding of disseminated tumor cells (DTCs) to bone, the survival of DTCs and microscopic metastases under dormancy, and the eventual outgrowth of overt metastases. In this review, we discuss the role of bone marrow niches in bone colonization. The emphasis is on complicated and dynamic nature of cancer cells-niche interaction, which may underpin the long-standing mystery of metastasis dormancy, and represent a therapeutic target for elimination of minimal residue diseases and prevention of life-taking, overt metastases.
Keywords: bone marrow niches; bone metastasis; disseminated tumor cells; hematopoietic stem cells.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest in this review.
Figures
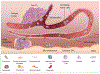

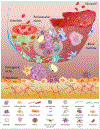
Similar articles
-
Mapping bone marrow niches of disseminated tumor cells.Sci China Life Sci. 2017 Oct;60(10):1125-1132. doi: 10.1007/s11427-017-9180-5. Epub 2017 Oct 11. Sci China Life Sci. 2017. PMID: 29027156
-
The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer.Oncotarget. 2016 Jul 5;7(27):41217-41232. doi: 10.18632/oncotarget.9251. Oncotarget. 2016. PMID: 27172799 Free PMC article.
-
Bone metastasis and the metastatic niche.J Mol Med (Berl). 2015 Nov;93(11):1203-12. doi: 10.1007/s00109-015-1329-4. Epub 2015 Aug 15. J Mol Med (Berl). 2015. PMID: 26275789 Free PMC article. Review.
-
Exploiting bone niches: progression of disseminated tumor cells to metastasis.J Clin Invest. 2021 Mar 15;131(6):e143764. doi: 10.1172/JCI143764. J Clin Invest. 2021. PMID: 33720051 Free PMC article. Review.
-
Bone marrow niches in the regulation of bone metastasis.Br J Cancer. 2021 Jun;124(12):1912-1920. doi: 10.1038/s41416-021-01329-6. Epub 2021 Mar 23. Br J Cancer. 2021. PMID: 33758331 Free PMC article. Review.
Cited by
-
Epigenetic dynamics in cancer stem cell dormancy.Cancer Metastasis Rev. 2020 Sep;39(3):721-738. doi: 10.1007/s10555-020-09882-x. Cancer Metastasis Rev. 2020. PMID: 32394305 Review.
-
Development of clinically relevant in vivo metastasis models using human bone discs and breast cancer patient-derived xenografts.Breast Cancer Res. 2019 Nov 29;21(1):130. doi: 10.1186/s13058-019-1220-2. Breast Cancer Res. 2019. PMID: 31783893 Free PMC article.
-
Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis.J Exp Clin Cancer Res. 2022 Oct 28;41(1):316. doi: 10.1186/s13046-022-02520-0. J Exp Clin Cancer Res. 2022. PMID: 36307871 Free PMC article. Review.
-
The role of calcium signaling in organotropic metastasis of cancer.Acta Pharmacol Sin. 2025 Jul;46(7):1801-1812. doi: 10.1038/s41401-025-01537-3. Epub 2025 Mar 25. Acta Pharmacol Sin. 2025. PMID: 40133629 Review.
-
NAT1 promotes osteolytic metastasis in luminal breast cancer by regulating the bone metastatic niche via NF-κB/IL-1B signaling pathway.Am J Cancer Res. 2020 Aug 1;10(8):2464-2479. eCollection 2020. Am J Cancer Res. 2020. PMID: 32905535 Free PMC article.
References
-
- Mundy GR (2002) Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 - PubMed
-
- Coleman RE (2006) Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 12, 6243s–6249s - PubMed
-
- Bubendorf L et al. (2000) Metastatic patterns of prostate cancer: An autopsy study of 1,589 patients. Hum. Pathol 31, 578–583 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

