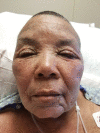
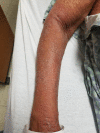
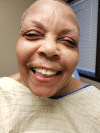
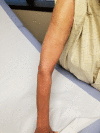
Hydrochlorothizide-induced acute generalised exanthematous pustulosis presenting with bilateral periorbital impetigo
- PMID: 30755424
- PMCID: PMC6381958
- DOI: 10.1136/bcr-2017-223528
Hydrochlorothizide-induced acute generalised exanthematous pustulosis presenting with bilateral periorbital impetigo
Abstract
Acute generalised exanthematous pustulosis (AGEP) is a severe cutaneous adverse reaction characterised by the appearance of erythematous plaques and papules with overlying non-follicular pinpoint pustules. Drugs are the cause of AGEP in approximately 90% of cases. The most common causes include anti-infective agents (aminopenicillins, quinolones, antibacterial sulfonamides and terbinafine), antimalarials and diltiazem. To the best of our knowledge, to date there has only been one report of hydrochlorothiazide-induced AGEP. There has never been a case report of losartan-induced AGEP. Here, we present a case of AGEP that is the second case purportedly caused by hydrochlorothiazide.
Keywords: cardiovascular system; dermatology; drug interactions unwanted effects / adverse reactions; drugs and medicines.
© BMJ Publishing Group Limited 2019. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Acute generalized exanthematous pustulosis.Chem Immunol Allergy. 2012;97:139-48. doi: 10.1159/000335625. Epub 2012 May 3. Chem Immunol Allergy. 2012. PMID: 22613859
-
Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore.Int J Dermatol. 2010 May;49(5):507-12. doi: 10.1111/j.1365-4632.2010.04313.x. Int J Dermatol. 2010. PMID: 20534083
-
Acute generalized exanthematous pustulosis associated with terbinafine: a case report.Cutan Ocul Toxicol. 2013 Oct;32(4):325-6. doi: 10.3109/15569527.2013.768256. Epub 2013 Feb 25. Cutan Ocul Toxicol. 2013. PMID: 23432048
-
Acute generalized exanthematous pustulosis: atypical presentations and outcomes.J Eur Acad Dermatol Venereol. 2015 Feb;29(2):209-214. doi: 10.1111/jdv.12721. Epub 2014 Sep 8. J Eur Acad Dermatol Venereol. 2015. PMID: 25201706 Review.
-
Acute generalised exanthematous pustulosis.Australas J Dermatol. 2012 May;53(2):87-92. doi: 10.1111/j.1440-0960.2011.00845.x. Epub 2011 Dec 29. Australas J Dermatol. 2012. PMID: 22571555 Review.
Cited by
-
Marginal corneal infiltrates as an ocular manifestation of acute generalized exanthematous pustulosis.Am J Ophthalmol Case Rep. 2023 Nov 9;32:101953. doi: 10.1016/j.ajoc.2023.101953. eCollection 2023 Dec. Am J Ophthalmol Case Rep. 2023. PMID: 38045987 Free PMC article.
-
Current Perspectives on Severe Drug Eruption.Clin Rev Allergy Immunol. 2021 Dec;61(3):282-298. doi: 10.1007/s12016-021-08859-0. Epub 2021 Jul 17. Clin Rev Allergy Immunol. 2021. PMID: 34273058 Free PMC article. Review.
-
Estradiol Valerate Tablets Caused Rare Severe Drug Eruption: The First Reported Case.Clin Cosmet Investig Dermatol. 2024 Oct 14;17:2277-2281. doi: 10.2147/CCID.S495269. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39430646 Free PMC article.
-
Drug Triggers and Clinic of Acute Generalized Exanthematous Pustulosis (AGEP): A Literature Case Series of 297 Patients.J Clin Med. 2022 Jan 13;11(2):397. doi: 10.3390/jcm11020397. J Clin Med. 2022. PMID: 35054090 Free PMC article. Review.
-
Multisystem organ failure secondary to acute generalised exanthematous pustulosis (AGEP) with atypical presentation resembling septic shock.BMJ Case Rep. 2022 Mar 1;15(3):e247040. doi: 10.1136/bcr-2021-247040. BMJ Case Rep. 2022. PMID: 35232738 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
