Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial
- PMID: 30755451
- PMCID: PMC6371944
- DOI: 10.1136/bmj.l236
Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial
Abstract
Objectives: To evaluate the effectiveness and safety at population scale of electronically delivered prescribing feedback and decision support interventions at reducing antibiotic prescribing for self limiting respiratory tract infections.
Design: Open label, two arm, cluster randomised controlled trial.
Setting: UK general practices in the Clinical Practice Research Datalink, randomised between 11 November 2015 and 9 August 2016, with final follow-up on 9 August 2017.
Participants: 79 general practices (582 675 patient years) randomised (1:1) to antimicrobial stewardship (AMS) intervention or usual care.
Interventions: AMS intervention comprised a brief training webinar, automated monthly feedback reports of antibiotic prescribing, and electronic decision support tools to inform appropriate prescribing over 12 months. Intervention components were delivered electronically, supported by a local practice champion nominated for the trial.
Main outcome measures: Primary outcome was the rate of antibiotic prescriptions for respiratory tract infections from electronic health records. Serious bacterial complications were evaluated for safety. Analysis was by Poisson regression with general practice as a random effect, adjusting for covariates. Prespecified subgroup analyses by age group were reported.
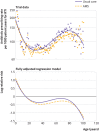
Results: The trial included 41 AMS practices (323 155 patient years) and 38 usual care practices (259 520 patient years). Unadjusted and adjusted rate ratios for antibiotic prescribing were 0.89 (95% confidence interval 0.68 to 1.16) and 0.88 (0.78 to 0.99, P=0.04), respectively, with prescribing rates of 98.7 per 1000 patient years for AMS (31 907 prescriptions) and 107.6 per 1000 patient years for usual care (27 923 prescriptions). Antibiotic prescribing was reduced most in adults aged 15-84 years (adjusted rate ratio 0.84, 95% confidence interval 0.75 to 0.95), with one antibiotic prescription per year avoided for every 62 patients (95% confidence interval 40 to 200). There was no evidence of effect for children younger than 15 years (adjusted rate ratio 0.96, 95% confidence interval 0.82 to 1.12) or people aged 85 years and older (0.97, 0.79 to 1.18); there was also no evidence of an increase in serious bacterial complications (0.92, 0.74 to 1.13).
Conclusions: Electronically delivered interventions, integrated into practice workflow, result in moderate reductions of antibiotic prescribing for respiratory tract infections in adults, which are likely to be of importance for public health. Antibiotic prescribing to very young or old patients requires further evaluation.
Trial registration: ISRCTN95232781.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the NIHR for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. Ethical approval: The protocol was approved by the NHS London-Dulwich research ethics committee (14/LO/1730) and by the CPRD independent scientific advisory committee (ISAC 14_130). Trial oversight was provided by independent trial steering and data monitoring committees. Each participating general practice gave written informed consent for participation.
Figures




Comment in
-
Improving outpatient antibiotic prescribing.BMJ. 2019 Feb 12;364:l289. doi: 10.1136/bmj.l289. BMJ. 2019. PMID: 30755402 No abstract available.
References
-
- Centres for Disease Control and Prevention. Antibiotic / antimicrobial resistance. Atlanta Georgia: CDC, 2018; https://www.cdc.gov/drugresistance/index.html.
-
- Venekamp RP, Sanders S, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2013;1:CD000219. - PubMed
