Perturbation of Macaque Supplementary Motor Area Produces Context-Independent Changes in the Probability of Movement Initiation
- PMID: 30755488
- PMCID: PMC6788817
- DOI: 10.1523/JNEUROSCI.2335-18.2019
Perturbation of Macaque Supplementary Motor Area Produces Context-Independent Changes in the Probability of Movement Initiation
Abstract
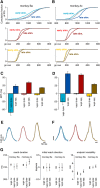
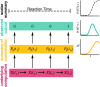
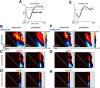
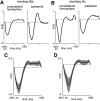
The contribution of the supplementary motor area (SMA) to movement initiation remains unclear. SMA exhibits premovement activity across a variety of contexts, including externally cued and self-initiated movements. Yet SMA lesions impair initiation primarily for self-initiated movements. Does SMA influence initiation across contexts or does it play a more specialized role, perhaps contributing only when initiation is less dependent on external cues? To address this question, we perturbed SMA activity via microstimulation at variable times before movement onset. Experiments used two adult male rhesus monkeys trained on a reaching task. We used three contexts that differed regarding how tightly movement initiation was linked to external cues. Movement kinematics were not altered by microstimulation. Instead, microstimulation induced a variety of changes in the timing of movement initiation, with different effects dominating for different contexts. Despite their diversity, these changes could be explained by a simple model where microstimulation has a stereotyped impact on the probability of initiation. Surprisingly, a unified model accounted for effects across all three contexts, regardless of whether initiation was determined more by external cues versus internal considerations. All effects were present for stimulation both contralateral and ipsilateral to the moving arm. Thus, the probability of initiating a pending movement is altered by perturbation of SMA activity. However, changes in initiation probability are independent of the balance of internal and external factors that establish the baseline initiation probability.SIGNIFICANCE STATEMENT The role of the supplementary motor area (SMA) in initiating movement remains unclear. Lesion experiments suggest that SMA makes a critical contribution only for self-initiated movements. Yet SMA is active before movements made under a range of contexts, suggesting a less-specialized role in movement initiation. Here, we use microstimulation to probe the role of SMA across a range of behavioral contexts that vary in the degree to which movement onset is influenced by external cues. We demonstrate that microstimulation produces a temporally stereotyped change in the probability of initiation that is independent of context. These results argue that SMA participates in the computations that lead to movement initiation and does so across a variety of contexts.
Keywords: macaque; microstimulation; reaching; supplementary motor area.
Copyright © 2019 the authors.
Figures








Similar articles
-
Changing directions of forthcoming arm movements: neuronal activity in the presupplementary and supplementary motor area of monkey cerebral cortex.J Neurophysiol. 1996 Oct;76(4):2327-42. doi: 10.1152/jn.1996.76.4.2327. J Neurophysiol. 1996. PMID: 8899607
-
Activation of the supplementary motor area (SMA) during performance of visually guided movements.Cereb Cortex. 2003 Sep;13(9):977-86. doi: 10.1093/cercor/13.9.977. Cereb Cortex. 2003. PMID: 12902397
-
Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate.J Neurophysiol. 1999 Jun;81(6):3065-77. doi: 10.1152/jn.1999.81.6.3065. J Neurophysiol. 1999. PMID: 10368421 Clinical Trial.
-
Bereitschaftspotential as an indicator of movement preparation in supplementary motor area and motor cortex.Ciba Found Symp. 1987;132:231-50. doi: 10.1002/9780470513545.ch14. Ciba Found Symp. 1987. PMID: 3322717 Review.
-
Exploring the Consistent Roles of Motor Areas Across Voluntary Movement and Locomotion.Neuroscientist. 2025 Jun;31(3):279-295. doi: 10.1177/10738584241263758. Epub 2024 Jul 23. Neuroscientist. 2025. PMID: 39041460 Free PMC article. Review.
Cited by
-
Continuous sensorimotor transformation enhances robustness of neural dynamics to perturbation in macaque motor cortex.Nat Commun. 2025 Apr 4;16(1):3213. doi: 10.1038/s41467-025-58421-1. Nat Commun. 2025. PMID: 40180984 Free PMC article.
-
A Theoretical Framework for Human and Nonhuman Vocal Interaction.Annu Rev Neurosci. 2022 Jul 8;45:295-316. doi: 10.1146/annurev-neuro-111020-094807. Epub 2022 Mar 22. Annu Rev Neurosci. 2022. PMID: 35316612 Free PMC article. Review.
-
Toward Precise Localization of Abnormal Brain Activity: 1D CNN on Single Voxel fMRI Time-Series.Front Comput Neurosci. 2022 Apr 27;16:822237. doi: 10.3389/fncom.2022.822237. eCollection 2022. Front Comput Neurosci. 2022. PMID: 35573265 Free PMC article.
-
Entrained neuronal activity to periodic visual stimuli in the primate striatum compared with the cerebellum.Elife. 2019 Sep 6;8:e48702. doi: 10.7554/eLife.48702. Elife. 2019. PMID: 31490120 Free PMC article.
-
A habenula-insular circuit encodes the willingness to act.Nat Commun. 2021 Nov 3;12(1):6329. doi: 10.1038/s41467-021-26569-1. Nat Commun. 2021. PMID: 34732720 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
